Hyaluronic acid (HA) is a biological polymer found ubiquitously in the extracellular matrix (ECM) of several tissues. Given the biocompatibility of HA, there is great interest in utilizing HA polymers to generate hydrogels for protein/drug delivery and tissue engineering applications.
The ability for a hydrogel to bind proteins (such as growth factors or cytokines) is an important design parameter to consider for protein-mediated tissue remodeling. For instance, incorporating sulfate groups in hydrogels increases the binding of proteins to a hydrogel through electrostatic interactions between sulfates and amino acids. Unfortunately, HA does not readily bind protein because the polymer does not have sulfate groups.
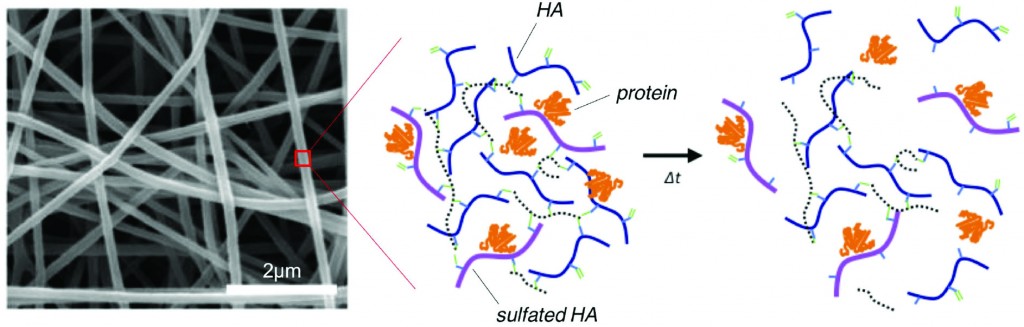
To address this limitation of HA, recent research in the Burdick Lab at the University of Pennsylvania focuses on designing HA polymers with sulfate groups to create robust hydrogels capable of protein binding and release. HA has an abundance of carboxylic acid groups along the polymer chain, making HA amenable to a variety of chemical modifications – including the addition of sulfate groups.
Researchers took advantage of carboxylic acid residues on HA to generate modified HA polymers with hydroxyethyl methacrylate (HEMA-HA). The methacrylate groups on HEMA-HA allow for free radical cross-linking of the polymers. The ester groups present in the cross-links also make the gel susceptible to hydrolytic degradation, which is an important feature for temporal release of bound protein from the hydrogel. Additionally, HEMA-HA polymers were allowed to react with SO3 to form sulfated HEMA-HA (HEMA-SHA). The resulting HEMA-HA and HEMA-SHA polymer products were characterized extensively with NMR and molecular weight/polydispersity measurements.
The binding affinities of HEMA-HA and HEMA-SHA were characterized using protein-binding assays of stromal cell-derived factor-1α (SDF-1α). Interestingly, SDF-1α binding to sulfated HA was comparable to heparin controls, and virtually no SDF-1α was bound to non-sulfated HA. The enhanced binding to HEMA-SHA is likely due to the increased negative charge from the sulfate groups on the HEMA-SHA (confirmed with zeta potential measurements).
After characterizing the binding efficiency of the polymers, HEMA-SHA and HEMA-HA were used to fabricate hydrogels. Using a 90%-10% blend of HEMA-HA and HEMA-SHA and a 100% solution HEMA-HA as a control, the polymer solutions were cross-linked into hydrogels using a redox initiator system for free radical cross-linking. Rheometry data show that the presence of sulfate groups in the hydrogel does not alter gel cross-linking kinetics and gel stiffness. Additionally, the sulfated HA hydrogel showed prolonged release of SDF-1α over time compared to the non-sulfated hydrogel control. These results show the ability to generate a robust sulfated HA hydrogel capable of controlled release of encapsulated protein.
This work describes a novel method to easily incorporate sulfate groups into HA hydrogels for enhanced binding and prolonged release of protein. The described technique is poised for use in future HA hydrogel designs for a wide array of drug delivery and tissue engineering applications.
Incorporation of sulfated hyaluronic acid macromers into degradable hydrogel scaffolds for sustained molecule delivery
Brendan P. Purcell, Iris L. Kim, Vanessa Chuo, Theodore Guenin, Shauna M. Dorsey and Jason A. Burdick
Biomater. Sci., 2014, Advance Article DOI: 10.1039/C3BM60227C
Brian Aguado is currently a Ph.D. Candidate and NSF Fellow in the Biomedical Engineering department at Northwestern University. He holds a B.S. degree in Biomechanical Engineering from Stanford University and a M.S. degree in Biomedical Engineering from Northwestern University. When he’s not in the lab, Brian enjoys traveling, cooking, swimming, and spending time with family and friends. Read more about Brian’s research publications here.
To keep up-to-date with all the latest research, sign-up to our RSS feed or Table of contents alert.