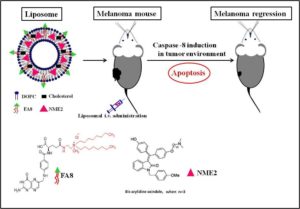
In cancers, rapid tumour growth is attributed to overexpression of anti-apoptotic proteins, inhibition or functional inefficiency of apoptotic proteases like caspases. Among caspases, caspase-8 signalling cascade is vital and interesting because of its ability to induce cell death by involving both mitochondria mediated intrinsic as well as death receptor (DR) – mediated extrinsic pathways. Notably, among ovarian cancer patients, tumors with low levels of caspase 8 are inherently resistant to chemotherapies. Incidentally, aggressive melanoma cells have functional expression of both Folate receptor (FR) on cell membrane and Estrogen receptor (ER) in cytoplasm. Stitching these basic facts one can deliver anti-cancer drugs, possibly targeting ER, using a liposomal system which will carry FR-targeting ligand to treat the aggressive melanoma cells.
In this present work, the Banerjee group used a hydrophobic drug molecule called NME2 (a recently developed ER-targeted anticancer drug for the treatment in breast cancer). Using a special FR-targeted liposome, the drug was successfully delivered to FR-moderately expressing melanoma cells.
The efficient targeting to FR-moderately expressed melanoma cells was accomplished by a new robust, cationic folate ligand named FA8. This efficiency of delivery is in stark contrast to other available FR-targeted liposomes which target only FR-over expressing cancer cells. The concoction of NME2 in FA8-associated liposome selectively induced caspase-8 expression-mediated apoptotic cell death in melanoma cancer cells (in vitro and in vivo). However, the drug in pristine state or in non-targeted liposome could not induce caspase-8 mediated apoptosis. Preliminarily, docetaxel, another potent anticancer drug, showed a similar result upon FA8-mediated delivery. Clearly, the given FR-targeted, liposomal delivery methodology indicated a change in mechanism of anticancer action of drug cargo and hence exemplified an interesting possibility to elude impending drug resistance (if any) against the given drug.
Tips from the authors:
1) In MDR cancers repurposing drug’s mechanistic pathway is essential, as acquired drug resistance is one of the major obstacles in fruitful cancer treatment.
2) The given FR-targeted formulation affected the change of mechanism of action of drug cargo (here, NME2) from non-caspase 8 to caspase-8 mediated apoptosis, thereby repurposing the apoptotic pathway of encapsulated drug.
3) The unique cationic lipid-conjugated folic acid based-ligand facilitated a) targeting to FR-moderately expressed melanoma cells; b) modification of mechanistic action of drug-cargo.
4) The liposomal delivery system with an FR-targeting ligand instigated an independent cell death pathway through the up-regulation of caspase-8 with subsequent cleavage of pro-survival factor RIP-1.
Article Link:
Cationic folate-mediated liposomal delivery of bis-arylidene oxindole induces efficient melanoma tumor regression Biomater. Sci., 2017, 5, 1898-1909
About the Webwriter:
 Dr. Sudip Mukherjee is a Webwriter for Biomaterials Science. He is currently a Postdoctoral Research Associate working alongside Dr. Omid Veiseh at the Department of Bioengineering at the Rice University. His research is involved in the development of advanced nanomaterials for drug/gene delivery in cancer theranostics, immunomodulatory applications & angiogenesis. He published a total of ~30 research articles/patents. He serves as International Advisory Board Member for ‘Materials Research Express‘, IOP Sciences. He is an associate member (AMRSC) of The Royal Society of Chemistry, UK. He serves as reviewer for several international journals like Chem Comm, J Mater Chem A, J Mater Chem B, Journal of Biomedical Nanotechnology, RSC Advances, IOP Nanotechnology etc.
Dr. Sudip Mukherjee is a Webwriter for Biomaterials Science. He is currently a Postdoctoral Research Associate working alongside Dr. Omid Veiseh at the Department of Bioengineering at the Rice University. His research is involved in the development of advanced nanomaterials for drug/gene delivery in cancer theranostics, immunomodulatory applications & angiogenesis. He published a total of ~30 research articles/patents. He serves as International Advisory Board Member for ‘Materials Research Express‘, IOP Sciences. He is an associate member (AMRSC) of The Royal Society of Chemistry, UK. He serves as reviewer for several international journals like Chem Comm, J Mater Chem A, J Mater Chem B, Journal of Biomedical Nanotechnology, RSC Advances, IOP Nanotechnology etc.