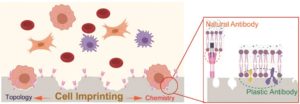
Sherlock Holmes solves mysterious cases often by identifying footprints and odor left by the suspects at the crime scenes. Scientists are now trying to trap notoriously rare circulating tumor cells (CTCs) in the bloodstream by imprinting their unique topologies and residual biomolecules for efficient early tumor detection.
The presence of CTCs is a dangerous signal for tumor progression, as they leak into the blood stream from the primary tumor and could potentially invade to other organs, causing metastasis. Unfortunately, they’re extremely rare and thus hard to identify, which poses great challenges for early tumor diagnosis. In a recent publication in Biomaterials Science, Gao et al from Shanghai Jiao Tong University developed a cell-imprinted biomimetic interface, which could intelligently recognize and efficiently capture CTCs, with an over 55% capture efficiency towards spiked MCF-7 cells, a human breast cancer cell line, from rabbit whole blood samples.
The mechanism behind is the synergy of “lock and key” topological and molecular interactions at the cell-biomaterials interface. Using target CTCs as an imprint template, they created a substrate mimicking their topologies and specific immunoaffinity. By leveraging soft lithography, hierarchical micro/nano-structures of CTCs could be recapitulated on an elastomer substrate (PDMS) to modulate cell adhesion behaviour: cell cytoskeleton staining showed that MCF-7 cells captured on the imprinted surface exhibited abundant filopodia and lamellipodia structures, while they showed an approximately spherical structure on flat substrate, indicating weak topographic interaction. Native proteins originating from CTC’s extracellular matrix (ECM) was anchored in the imprinted substrates providing artificial recognition receptors for selective CTC capture. This is supported by the significantly higher MCF-7 cells capture efficiency on MCF-7 imprinted substrates comparing to HeLa-cell (a human-cervical-cancer cell line) imprinted ones. Anti-EpCAM, a natural antibody, was also introduced to accelerate the CTC-substrate interactions. These interactions turned out to play a decisive role in cell capture, followed by that with plastic receptors.
By recapitulating the topological and chemical microenvironment of CTCs, this biomimetic cell-imprinted substrates demonstrate potential for rare cancer cell capture. Considering the significant roles of tissue ECM stiffness and viscoelasticity in metastasis, and the strong integrin-mediated focal adhesion at the CTCs-biomaterial interface, it might be interesting to incorporate mechanical signals into the interface design in the future for enhanced CTCs capture.
Read the full article for FREE until the 9th October!
Cell-imprinted biomimetic interface for intelligent recognition and efficient capture of CTCs, by Su Gao, Shuangshuang Chen and Qinghua Lu
About the web writer
Zhenwei Ma is currently a Ph.D. candidate in Mechanical Engineering at McGill University. He holds a M.E. degree in Chemical Engineering at McGill University and a B.E. degree in Chemical Engineering from Sichuan University. Find out more about him here.