Chisvert & Salvador, Anal. Methods, 2013, 5,
The second issue of Analytical Methods is online now, and it features papers from a themed issue on Cosmetic Ingredients: from the cosmetic to the human body and the environment. Read all about this topic in the Editorial from Guest Editors Alberto Chisvert and Amparo Salvador from the University of Valencia, Spain. The outside front cover comes from our guest editors and represents the themes from this issue.
Cosmetic ingredients: from the cosmetic to the human body and the environment
Alberto Chisvert and Amparo Salvador
Anal. Methods, 2013, 5, 309-310
DOI: 10.1039/C2AY90060B

Chen et al., Anal. Methods, 2013, 5,
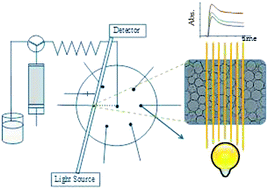
On the inside front cover we have an image from Huanwen Chen at East China Institute of Technology, and colleagues from China. In their minireview they have covered the technical developments and applications of extractive electrospray ionization mass spectrometry for the rapid analysis of cosmetic products.
Extractive electrospray ionization mass spectrometry for direct characterization of cosmetic products
Xinglei Zhang, Nannan Wang, Yafei Zhou, Yan Liu, Jinghua Zhang and Huanwen Chen
Anal. Methods, 2013, 5, 311-315
DOI: 10.1039/C2AY25876E
The cover articles will be free to read for 6 weeks.
There are also a number of interesting review papers and exciting research articles on a variety of issues including: analysis of perfumes, surfactants and UV filters in the environment, and microbial contamination of cosmetics. Take a look at a selection of these papers below, they will be free until January 18th.
Fragrances: from essential oils to the human body and atmospheric aerosols
Lai-Hao Wang
Anal. Methods, 2013, 5, 316-322
DOI: 10.1039/C2AY25980J
Current trends in liquid–liquid and solid–liquid extraction for cosmetic analysis: a review
N. Cabaleiro, I. de la Calle, C. Bendicho and I. Lavilla
Anal. Methods, 2013, 5, 323-340
DOI: 10.1039/C2AY25830G
Analytical methods for the characterization and determination of nonionic surfactants in cosmetics and environmental matrices
M. Beneito-Cambra, J. M. Herrero-Martínez and G. Ramis-Ramos
Anal. Methods, 2013, 5, 341-354
DOI: 10.1039/C2AY25847A
A novel outlook on detecting microbial contamination in cosmetic products: analysis of biomarker volatile compounds by solid-phase microextraction gas chromatography-mass spectrometry
Liquid chromatography-tandem mass spectrometry for the multi-residue analysis of organic UV filters and their transformation products in the aquatic environment
Pablo Gago-Ferrero, M. Silvia Díaz-Cruz and Damià Barceló
Anal. Methods, 2013, 5, 355-366
DOI: 10.1039/C2AY26115D
A solid-phase extraction liquid chromatography-tandem mass spectrometry method for the percutaneous absorption assessment of 3-(4′-methylbenzylidene)camphor via human urine analysis
Zacarías León-González, Alberto Chisvert, Isabel Fernández and Amparo Salvador
Anal. Methods, 2013, 5, 367-375
DOI: 10.1039/C2AY25490E
Measurement of iodide and caffeine content in cellulite reduction cosmetic products sold in the European market
Emilia Marchei, Daniela De Orsi, Carmine Guarino, Stefano Dorato, Roberta Pacifici and Simona Pichini
Anal. Methods, 2013, 5, 376-383
DOI: 10.1039/C2AY25761K
A novel outlook on detecting microbial contamination in cosmetic products: analysis of biomarker volatile compounds by solid-phase microextraction gas chromatography-mass spectrometry
Gerardo Alvarez-Rivera, Trinidad De Miguel, Maria Llompart, Carmen Garcia-Jares, Tomas Gonzalez Villa and Marta Lores
Anal. Methods, 2013, 5, 384-393
DOI: 10.1039/C2AY25833A
Take a look at all the papers from this themed issue on Cosmetic Ingredients here.
Comments Off on Analytical Methods Issue 2: Cosmetic Ingredients