This month sees the following articles in Organic & Biomolecular Chemistry that are in the top ten most accessed:
Synthesis of the anti-influenza agent (-)-oseltamivir free base and (-)-methyl 3-epi-shikimate
Varun Rawat, Soumen Dey and Arumugam Sudalai
Org. Biomol. Chem., 2012, 10, 3988-3990
DOI: 10.1039/C2OB25635E
Thiol–yne coupling: revisiting old concepts as a breakthrough for up-to-date applications
Alessandro Massi and Daniele Nanni
Org. Biomol. Chem., 2012, 10, 3791-3807
DOI: 10.1039/C2OB25217A
Cu(I)-catalyzed annulation for the synthesis of substituted naphthalenes using o-bromobenzaldehydes and ß-ketoesters as substrates
Chandi C. Malakar, Kavitha Sudheendran, Hans-Georg Imrich, Sabine Mika and Uwe Beifuss
Org. Biomol. Chem., 2012, 10, 3899-3905
DOI: 10.1039/C2OB06963F
A convenient and mild chromatography-free method for the purification of the products of Wittig and Appel reactions
Peter A. Byrne, Kamalraj V. Rajendran, Jimmy Muldoon and Declan G. Gilheany
Org. Biomol. Chem., 2012, 10, 3531-3537
DOI: 10.1039/C2OB07074J
Copper(II)-mediated oxidative cyclization of enamides to oxazoles
Alison E. Wendlandt and Shannon S. Stahl
Org. Biomol. Chem., 2012, 10, 3866-3870
DOI: 10.1039/C2OB25310K
Synthesis of carbazolones and 3-acetylindoles via oxidative C–N bond formation through PIFA-mediated annulation of 2-aryl enaminones
Xu Ban, Yan Pan, Yingfu Lin, Songqing Wang, Yunfei Du and Kang Zhao
Org. Biomol. Chem., 2012, 10, 3606-3609
DOI: 10.1039/C2OB25348H
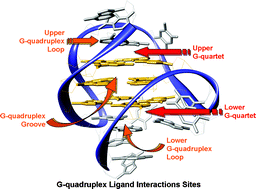
Water soluble extended naphthalene diimides as pH fluorescent sensors and G-quadruplex ligands
Filippo Doria, Matteo Nadai, Giovanna Sattin, Luca Pasotti, Sara N. Richter and Mauro Freccero
Org. Biomol. Chem., 2012, 10, 3830-3840
DOI: 10.1039/C2OB07006E
Tunnelling control of chemical reactions – the organic chemist’s perspective
David Ley, Dennis Gerbig and Peter R. Schreiner
Org. Biomol. Chem., 2012, 10, 3781-3790
DOI: 10.1039/C2OB07170C
Enantioselective synthesis of bio-relevant 3,5-diaryl pyrazolines
Olivier Mahé, Isabelle Dez, Vincent Levacher and Jean-François Brière
Org. Biomol. Chem., 2012, 10, 3946-3954
DOI: 10.1039/C2OB25227A
Development of strong Brønsted base catalysis: catalytic direct-type Mannich reactions of non-activated esters via a product-base mechanism
Yasuhiro Yamashita, Hirotsugu Suzuki and Shū Kobayashi
Org. Biomol. Chem., 2012, Advance Article
DOI: 10.1039/C2OB25522G
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Organic & Biomolecular Chemistry? Then why not submit to us today or alternatively email us your suggestions.
Comments Off on Top ten most accessed article in April
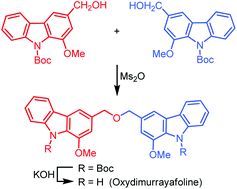











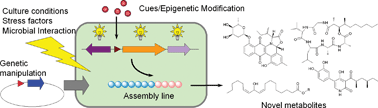 Triggering cryptic natural product biosynthesis in microorganisms
Triggering cryptic natural product biosynthesis in microorganisms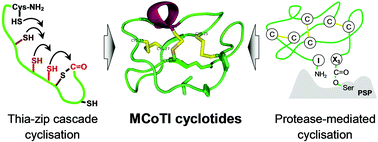 Chemical and biomimetic total syntheses of natural and engineered MCoTI cyclotides
Chemical and biomimetic total syntheses of natural and engineered MCoTI cyclotides

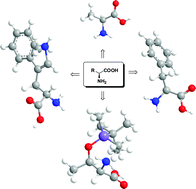 Primary amino acids: privileged catalysts in enantioselective organocatalysis
Primary amino acids: privileged catalysts in enantioselective organocatalysis