Cancer researchers in the US and China have combined the turmeric spice pigment curcumin and the drug thalidomide to create hybrid compounds that can kill multiple myeloma cells.
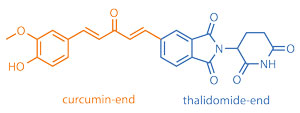 Multiple myeloma is the second most common type of blood cancer, killing 20% of affected patients each year. The drug thalidomide, banned after causing birth defects when given during pregnancy in the 1950s, was recently rediscovered and approved for the treatment of multiple myeloma. Thalidomide works by disturbing the microenvironment of tumour cells in bone marrow. However, it disintegrates in the body. Curcumin, a yellow pigment from the common spice turmeric, is also active against cancers, including myeloma, but is limited by its poor water solubility.
Multiple myeloma is the second most common type of blood cancer, killing 20% of affected patients each year. The drug thalidomide, banned after causing birth defects when given during pregnancy in the 1950s, was recently rediscovered and approved for the treatment of multiple myeloma. Thalidomide works by disturbing the microenvironment of tumour cells in bone marrow. However, it disintegrates in the body. Curcumin, a yellow pigment from the common spice turmeric, is also active against cancers, including myeloma, but is limited by its poor water solubility.
Shijun Zhang at Virginia Commonwealth University, US, and colleagues, have synthesised compounds combining structural features from both thalidomide and curcumin. ‘The hybrids have enhanced solubility, and higher toxicity against myeloma cells than curcumin, thalidomide, or a mixture of both,’ explains Zhang, ‘so our design rational is going in the right direction.’ Zhang says the hybrids kill myeloma cells through combined mechanisms of action that include the generation of reactive oxygen species and cell cycle inhibition.
Read the full story on Chemistry World.
K Liu et al, Org. Biomol. Chem., 2013, DOI: 10.1039/c3ob40595h
Free to access for 4 weeks!