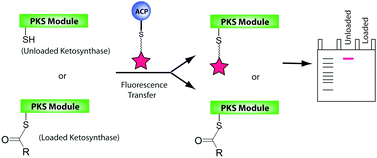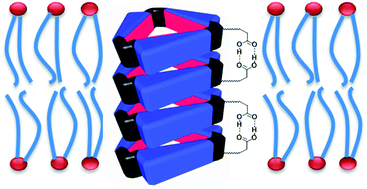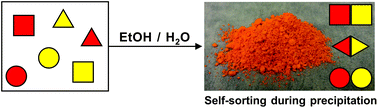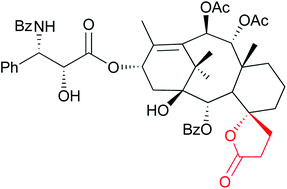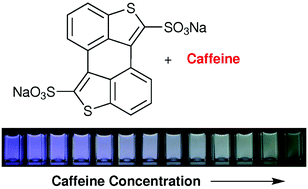
The system’s sensitivity and selectivity are higher than those of current synthetic caffeine sensors, so much so that caffeine can be detected in the sub-millimolar concentration range, the researchers claim. The team prepared sensor test strips on which a change in emission colour on addition of caffeine was easy to detect by the naked eye.
A ratiometric fluorescence sensor for caffeine
Nicolas Luisier, Albert Ruggi, Stephan N. Steinmann, Laurane Favre, Nicolas Gaeng, Clémence Corminboeuf and Kay Severin
DOI: 10.1039/C2OB26117K