Posted on behalf of Dr Liana Allen, guest web writer for Organic & Biomolecular Chemistry.
Antibiotics are drug compounds used to treat infections caused by bacteria, such as tuberculosis and some forms of meningitis. The processes involved in bacteria cell reproduction are highly complicated and many different targets along the biological pathway of bacterial cell replication have been identified as potential opportunities to disrupt bacterial growth. Antibacterial drugs can be described as ‘bactericidal’ if they kill the bacterial cells by inhibition of bacteria cell wall or cell membrane synthesis, or by interfering with an essential bacterial enzyme. Those which target bacterial protein synthesis can be described as ‘bacteriostatic’ as they stop bacteria from reproducing but otherwise do not harm them.
One specific mode of antibacterial action is interaction with DNA gyrase, which is an enzyme involved in the unwinding of double stranded DNA helices during DNA replication.1 DNA gyrase is an enzyme which is not present in humans, making it an especially good target for antibiotics. One way in which antibacterial molecules interact with DNA gyrase is through stabilization of a DNA-gyrase covalent complex which is formed during the DNA replication process. The result of this interaction is the release of ‘broken DNA’, which kills the organism. For this reason, antibacterial agents which behave in this way are referred to as DNA gyrase poisons.
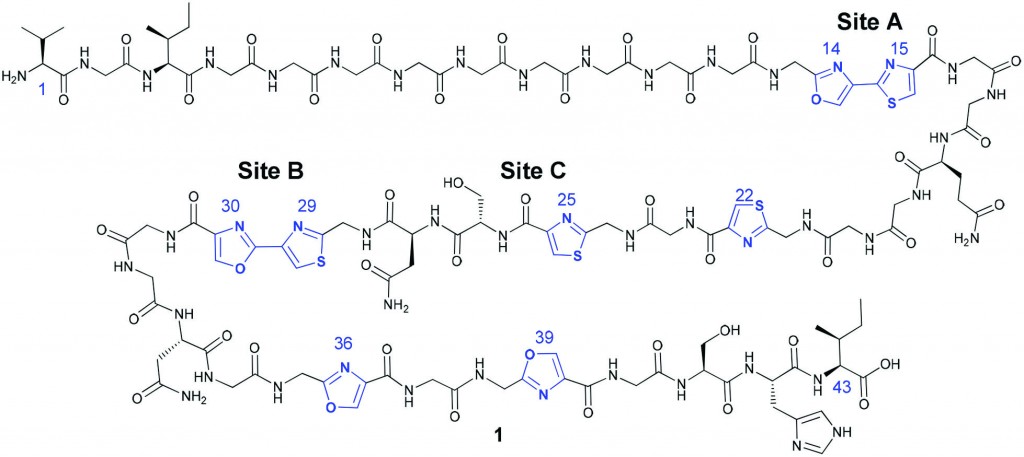
Microcin B17 (MccB17)
Microcin B17 (MccB17) is a peptide which displays antibacterial action and is thought to act as a DNA gyrase poison, but unfortunately, the full MccB17 peptide is not a suitable drug candidate due to its poor physiochemical properties. MccB17 is particularly interesting as the mature peptide contains some unusual hetrocyclic units.
In this paper, Katrina A. Jolliffe, Richard J. Payne and colleagues aimed to investigate the structure-activity relationship of MccB17 in order to determine exactly which parts of the peptide are needed for the antibacterial action and to develop a simplified DNA gyrase poison which would be a good lead candidate for antibacterial drug discovery. Using their own synthetic methodology,2 they prepared a range of full length and truncated analogues of MccB17, then tested their abilities to act as DNA gyrase poisons. They then compared the results with a known, potent DNA gyrase poison, ciprofloxacin. Many of the prepared analogues showed activity very similar to the native peptide; however some of the truncated variants actually performed better in this test. These promising results now enable more detailed investigation into the mechanism of bactericidal action of MccB17. In a second series of experiments, the peptides were tested for their antibacterial activity against two strains of E. coli. In this test it was the full length native MccB17 which gave the best results, suggesting that, even though truncated analogues can still exhibit DNA gyrase poisoning activity, the full length sequence would still currently be needed for bactericidal activity in vivo.
To read more, see;
Synthesis of Full Length and Truncated Microcin B17 Analogues as DNA Gyrase Poisons
R. E. Thompson, F. Collin, A. Maxwell, K. A. Jolliffe and R. J. Payne,
Organic & Biomolecular Chemistry, 2014, DOI: 10.1039/C3OB42516A.
Free to access until 7th March
References
1 Gore, J., Bryant, Z., Stone, M. D., Nöllmann, M., Cozzarelli, N.R., Bustamante, C. Nature, 2006, 439, 100.
2 Thompson, R. E., Jolliffe, K. A., Payne, R. J., Org. Lett., 2011, 13, 680.
 Dr C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.
Dr C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.












 Dr C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.
Dr C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.