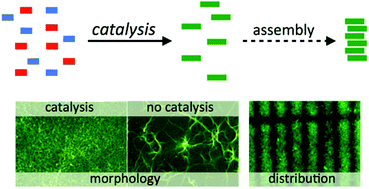
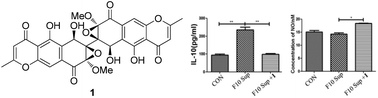
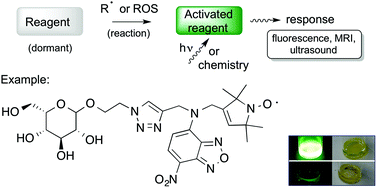
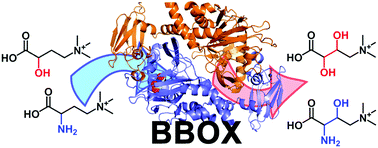The following Organic & Biomolecular Chemistry articles have all been recommened by the reviewers of the articles as being particularly interesting or particularly significant research. These have all been made free to access until 31st December 2014. The order they appear in the list holds no special meaning or ranking.
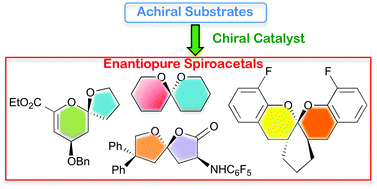
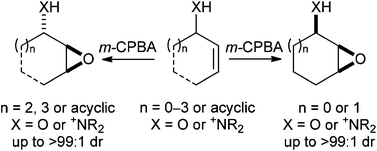
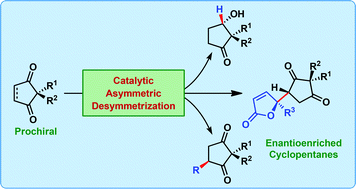
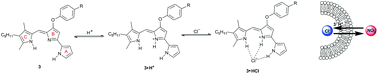
Catalytic asymmetric desymmetrization approaches to enantioenriched cyclopentanes
Madhu Sudan Manna and Santanu Mukherjee
DOI: 10.1039/C4OB01649A, Perspective
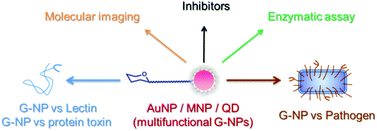
Corrin-based chemosensors for the ASSURED detection of endogenous cyanide
Felix Zelder and Lucas Tivana
DOI: 10.1039/C4OB01889C, Perspective
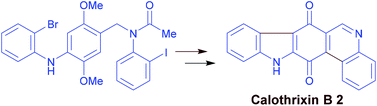
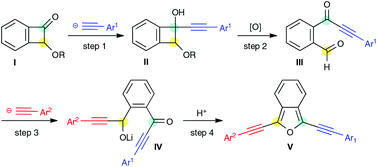
An efficient synthetic route to 1,3-bis(arylethynyl)isobenzofuran using alkoxybenzocyclobutenone as a reactive platform
Kenta Asahina, Suguru Matsuoka, Ryosuke Nakayama and Toshiyuki Hamura
DOI: 10.1039/C4OB02012J, Communication
Cationic azacryptands as selective three-way DNA junction binding agents
Jana Novotna, Aurelien Laguerre, Anton Granzhan, Marc Pirrotta, Marie-Paule Teulade-Fichou and David Monchaud
DOI: 10.1039/C4OB01846J, Paper
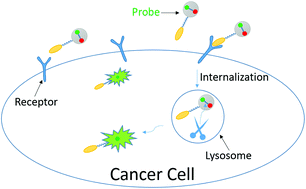
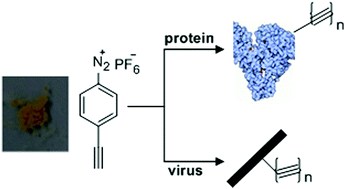
An efficient reagent for covalent introduction of alkynes into proteins
Jie Zhang, Dejun Ma, Dawei Du, Zhen Xi and Long Yi
DOI: 10.1039/C4OB01873G, Communication
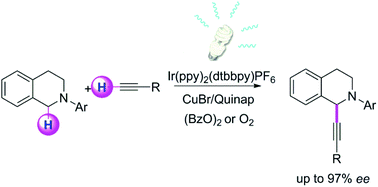
Efficient merging of copper and photoredox catalysis for the asymmetric cross-dehydrogenative-coupling of alkynes and tetrahydroisoquinolines
Inna Perepichka, Soumen Kundu, Zoë Hearne and Chao-Jun Li
DOI: 10.1039/C4OB02138J, Paper
Naphthalene diimides as red fluorescent pH sensors for functional cell imaging
Filippo Doria, Marco Folini, Vincenzo Grande, Graziella Cimino-Reale, Nadia Zaffaroni and Mauro Freccero
DOI: 10.1039/C4OB02054E, Paper
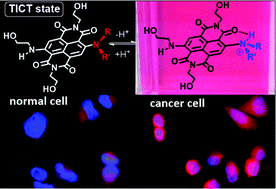
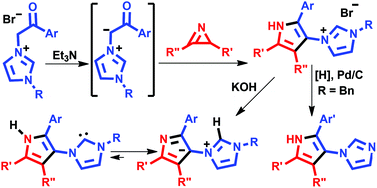
S. Bartocci, D. Mazzier, A. Moretto and M. Mba
DOI: 10.1039/C4OB02102A, Communication
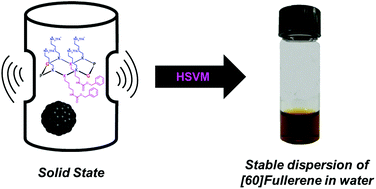
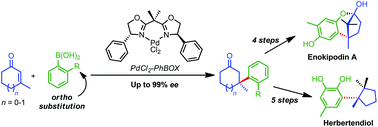
Muhammet Tanc, Fabrizio Carta, Andrea Scozzafava and Claudiu T. Supuran
DOI: 10.1039/C4OB02155J, Communication
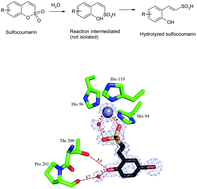
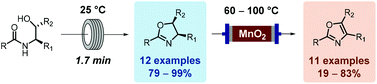
Steffen Glöckner, Duc N. Tran, Richard J. Ingham, Sabine Fenner, Zoe E. Wilson, Claudio Battilocchio and Steven V. Ley
DOI: 10.1039/C4OB02105C, Paper
From themed collection Recent Advances in Flow Synthesis and Continuous Processing












![Stereoselective intermolecular [2 + 2]-photocycloaddition reactions of maleic anhydride: stereocontrolled and regiocontrolled access to 1,2,3-trifunctionalized cyclobutanes Stereoselective intermolecular [2 + 2]-photocycloaddition reactions of maleic anhydride](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C4OB01383B)
![Relative contractile motion of the rings in a switchable palindromic [3]rotaxane in aqueous solution driven by radical-pairing interactions](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C4OB01228C)