On the front cover is this HOT Emerging Areaarticle from Peter R. Schreiner and co-workers (Justus-Liebig University, Germany) who discuss quantum mechanical tunnelling and provide an overview of the importance of tunnelling in organic chemical reactions.
On the front cover is this HOT Emerging Areaarticle from Peter R. Schreiner and co-workers (Justus-Liebig University, Germany) who discuss quantum mechanical tunnelling and provide an overview of the importance of tunnelling in organic chemical reactions.
Discussion includes:
- a brief history of tunnelling
- hydrogen tunnelling
- carbon tunnelling
- heteroatom tunnelling
Tunnelling control of chemical reactions – the organic chemist’s perspective
David Ley, Dennis Gerbig and Peter R. Schreiner
Org. Biomol. Chem., 2012, 10, 3781-3790
DOI: 10.1039/C2OB07170C
 On the inside front cover is this Communication, from Ying-Yeung Yeung and colleagues at National University of Singapore, where a facile and highly enantioselective route to 2-substituted 3-bromopyrrolidines is presented. The authors demonstrate how these can be reached via the bromo-aminocyclisation of 1,2-disubstituted olefinic amides using amino-thiocarbamates as a catalyst. This is also part of our growing Organocatalysis web collection.
On the inside front cover is this Communication, from Ying-Yeung Yeung and colleagues at National University of Singapore, where a facile and highly enantioselective route to 2-substituted 3-bromopyrrolidines is presented. The authors demonstrate how these can be reached via the bromo-aminocyclisation of 1,2-disubstituted olefinic amides using amino-thiocarbamates as a catalyst. This is also part of our growing Organocatalysis web collection.
A highly enantioselective approach towards 2-substituted 3-bromopyrrolidines
Jie Chen, Ling Zhou and Ying-Yeung Yeung
Org. Biomol. Chem., 2012, 10, 3808-3811
DOI: 10.1039/C2OB25327E
Both of these cover articles will be free to access for the next 6 weeks.
Also in this issue:
A perspectiveby Alessandro Massi and Daniele Nanni:
Thiol–yne coupling: revisiting old concepts as a breakthrough for up-to-date applications
And 2 HOT articles that are still free to access for another 3 weeks
Diastereoselective alkylation reactions of 1-methylcyclohexa-2,5-diene-1-carboxylic acid
Mark C. Elliott et al.
Glycosylated diazeniumdiolate-based oleanolic acid derivatives: synthesis, in vitro and in vivo biological evaluation as anti-human hepatocellular carcinoma agents
Yihua Zhang et al.
View the entire issue HERE, it’s full of lots of great content!











![GA[6]](https://blogs.rsc.org/ob/files/2012/04/GA6-300x103.gif)


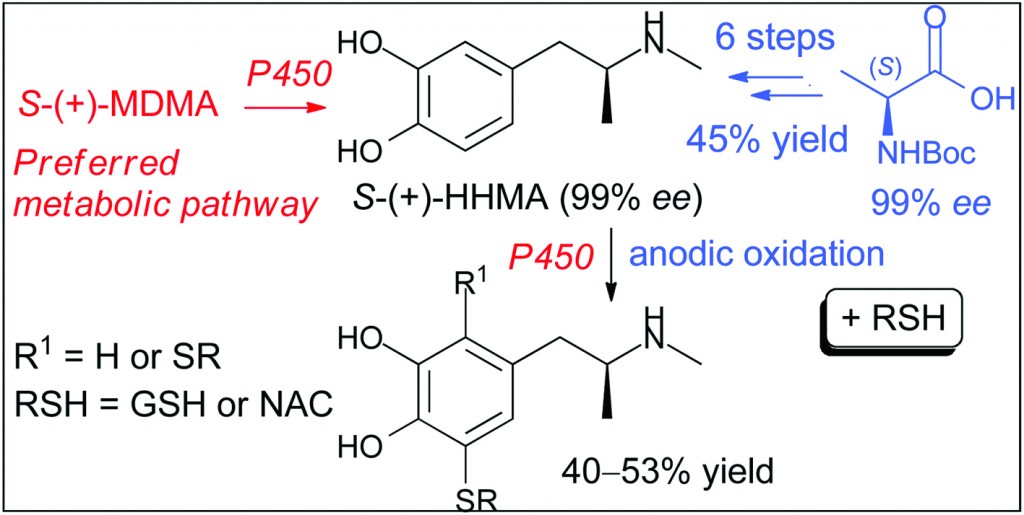 MDMA, also known as “ecstasy”, is a psychoactive drug with selective neurotoxic potential toward brain serotonin neurons. Despite intensive research, the precise mechanism by which MDMA selectively damages brain neurons in most species remains unknown. One hypothesis is that MDMA neurotoxicity may at least partially be a consequence of its metabolism. In particular, O-demethylenated MDMA metabolites such as N-methyl-α-methyldopamine (HHMA) have been postulated to serve as precursors for toxic catechol–thioether conjugates.
MDMA, also known as “ecstasy”, is a psychoactive drug with selective neurotoxic potential toward brain serotonin neurons. Despite intensive research, the precise mechanism by which MDMA selectively damages brain neurons in most species remains unknown. One hypothesis is that MDMA neurotoxicity may at least partially be a consequence of its metabolism. In particular, O-demethylenated MDMA metabolites such as N-methyl-α-methyldopamine (HHMA) have been postulated to serve as precursors for toxic catechol–thioether conjugates.