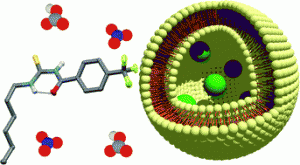
Acylthioureas that can act as ion transporters across cell membranes may lead to novel treatments for cystic fibrosis and associated conditions.
Inorganic anions such as chloride play a crucial role in biological systems, and the mechanisms behind their transport and regulation are still to be fully understood. Proteins embedded in the lipid bilayer regulate these transport processes and carry anions across cell membranes. When these processes are defective, then channelopathic diseases such as cystic fibrosis can develop.
Synthetic anion transporters based on small molecules may serve as replacements for faulty transport proteins, and may therefore find use in the treatment of such diseases.
Professor Philip Gale and his research group at the University of Southampton have been looking into synthetic membrane transporters for anions based on thiourea scaffolds.
In this HOT Article, Prof. Gale and co-workers discuss the synthesis of a series of acylthioureas and their potential as anion transporters in POPC lipid bilayers. They have discovered that these molecules function effectively as anion antiporters, but that the incorporation of lipophilic moieties leads to a reduction in their efficiency. This is due to an intramolecular hydrogen bond that forms and shields the binding site from interactions with water.
It is clear from these results that striking a balance between hydro- and lipo-philicity of the anion transporter is of crucial consideration in their efficiency. Furthermore, the intramolecular hydrogen bonding behaviour of these molecules may be of use in enabling more hydrophilic moieties to be incorporated in future transporter design. It is hoped that this information will eventually lead to the development of new transporters for medicinal applications.
Acylthioureas as anion transporters: the effect of intramolecular hydrogen bonding
Cally J. E. Haynes, Nathalie Busschaert, Isabelle L. Kirby, Julie Herniman, Mark E. Light, Neil J. Wells, Igor Marques, Vítor Félix and Philip A. Gale
Org. Biomol. Chem., 2013, DOI: 10.1039/C3OB41522H