For centuries, natural products have been linked to medicine through traditional remedies and since played an important role in drug discovery.
Despite competition from other drug discovery methods, natural products have provided their fair share of clinical candidates and commercial drugs. Furthermore, the isolation, synthesis and biological evaluation of natural products often lead to lasting impressions in science.
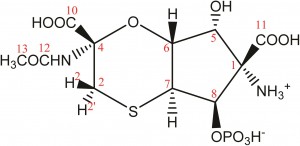
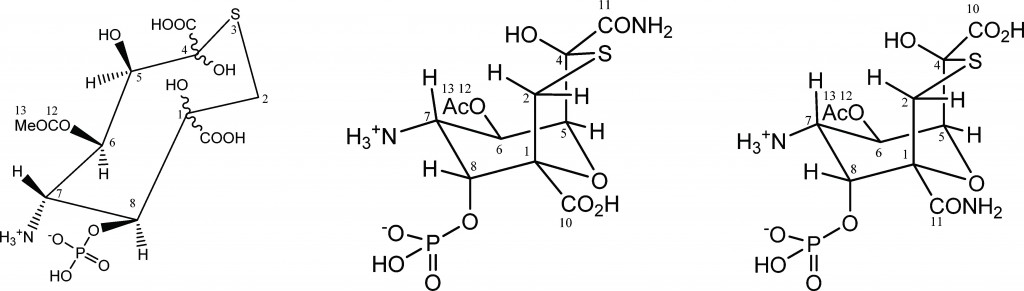
In a recent study lead by Dr Abil Aleiv of the University College London, the structure of the known natural product tagetitoxin has been revised based on a detailed analysis of newly acquired NMR and MS data. The group employed 2D 1H–13C HMBC correlations and long-range JCH couplings in conjunction with computational analysis to correlate JCH couplings with predicted values.
For several years, the structure of tagetitoxin remained a mystery. First identified in 1981 by Mitchell, the structure was only partially characterized by MS and was proposed to be an 8-membered heteroatomic ring. Revised structures have since been published by Mitchel (1989), Vassylyev (2005) and Gronwald (2005). Despite all these efforts, conflicting results and incomplete analyses resulted in the absolute configuration remaining undetermined.
Early analysis of complex structures was generally difficult as spectrometers were relatively insensitive and experiments were performed at low-fields strengths. Through the increasing prevalence and utility of modern 2D NMR experiments in the past decade, NMR has become a powerful and enabling tool for structure elucidation and confirmation.
In addition, the key to Dr Aliev’s findings lies in confirming the purity of the tagetitoxin sample the group had acquired. They noted that the compound gradually decomposed in aqueous solutions if left for prolonged periods of time, which they suspect led to additional peaks being observed in previously reported NMR spectra.
This exciting work showcases the importance of technical advances in determining the structure of biologically active natural products with greater ease and confidence. As a result, advances in lead development and the identification of important families of pharmacophores for drug discovery can be attained with greater efficiency, which may contribute to a revival of interest in natural products for drug discovery purposes.
To find out more see:
The structure of tagetitoxin
Abil E. Aliev, Kersti Karu, Robin E. Mitchell and Michael J. Porter
DOI: 10.1039/c5ob02076j
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at The University of Toronto. Her research is centred on the synthesis of kinetically amphoteric molecules, which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.