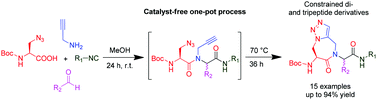
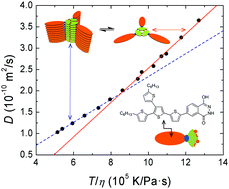
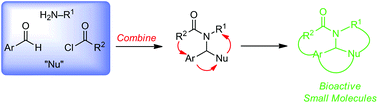
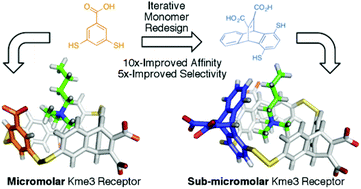
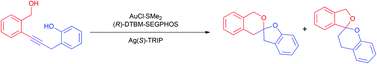Advances in hypervalent iodine are flourishing1 from the classic non-toxic Dess-Martin periodinane oxidation to synthetic reagents for elaborate natural products2. New types of both iodine(III) and iodine(V) compounds are constantly being discovered for ever increasing applications.
Iodonium salts, classified as iodine(III) compounds, are notoriously electrophilic. Those with two carbon ligands like alkynyl(aryl)iodonium salts react with a nucleophile which couples with the alkyne. Overall, the reaction seemingly pieces together two nucleophilic entities.
The remaining aryl iodide in the balanced equation seems to be a “spectator” since it is absent in the product, but in this recent (open access) paper Hamnett and Moran sought to investigate whether that is truly the case.
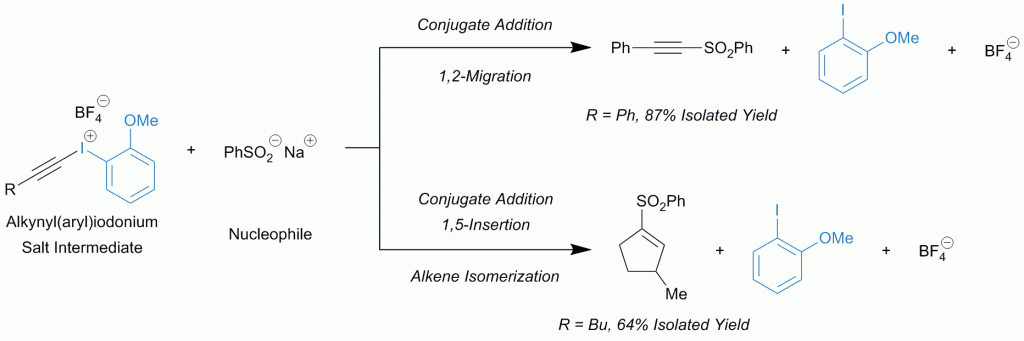
They improved the preparation of alkynyl(aryl)iodonium tosylates and systematically applied them to two model reactions, finding that the 2-anisyl ligand provided the highest yields.
It significantly out-performed the 3-anisyl derivative, suggesting that the key to success is the inherent stability of the 2-anisyl iodonium salt with the methoxy group ideally situated for coordination to the iodine.
Discovering the effectiveness of the 2-anisyl ligand in iodonium salts could have implications for more efficient transformations involving hypervalent iodine. More broadly, the work shows the importance of optimizing reactions across all variables and highlighting that spectators may not always be simple bystanders.
To find out more see:
Improving alkynyl(aryl)iodonium salts: 2-anisyl as a superior aryl group
David J. Hamnett and Wesley J. Moran
DOI: 10.1039/C4OB00556B
1. M. S. Yusubov, A. V. Maskaev and V. V. Zhdankin,
ARKIVOC, 2011,
i, 370–409.
2. D. J. Wardrop and J. Fritz,
Org. Lett., 2006,
8, 3659–3662.
Posted on behalf of Jennifer Lee, guest web writer for Organic & Biomolecular Chemistry
 Jennifer Lee is currently a Ph.D. candidate in Dr. George Kraus’ organic chemistry lab at Iowa State University. Her research focuses on designing methodologies to transform carbohydrate-derived biomass into biorenewable commodity and specialty chemicals. The creation of a versatile platform technology led to diverse and industrially-relevant aromatic compounds to work toward a more sustainable future.
Jennifer Lee is currently a Ph.D. candidate in Dr. George Kraus’ organic chemistry lab at Iowa State University. Her research focuses on designing methodologies to transform carbohydrate-derived biomass into biorenewable commodity and specialty chemicals. The creation of a versatile platform technology led to diverse and industrially-relevant aromatic compounds to work toward a more sustainable future.
 A new sensing system that changes colour to indicate if a cassava-based foodstuff is safe to eat by checking for hydrogen cyanide has been devised by researchers in Switzerland and Mozambique.
A new sensing system that changes colour to indicate if a cassava-based foodstuff is safe to eat by checking for hydrogen cyanide has been devised by researchers in Switzerland and Mozambique.