 To date, more than 320 new alkaloids have been isolated from the evergreen plants of the Daphniphyllum genus. In ancient times, extracts of the bark and leaves of Daphniphyllum plants were used in Chinese herbal medicines to cure minor ailments and treat pain. Recent studies have discerned that the Daphniphyllum class of alkaloid displays significant and varying biological activity, including anticancer, antioxidant and vasorelaxant activities. However, their unique polycyclic architectures containing multiple quaternary stereocenters render these alkaloids synthetically challenging.
To date, more than 320 new alkaloids have been isolated from the evergreen plants of the Daphniphyllum genus. In ancient times, extracts of the bark and leaves of Daphniphyllum plants were used in Chinese herbal medicines to cure minor ailments and treat pain. Recent studies have discerned that the Daphniphyllum class of alkaloid displays significant and varying biological activity, including anticancer, antioxidant and vasorelaxant activities. However, their unique polycyclic architectures containing multiple quaternary stereocenters render these alkaloids synthetically challenging.
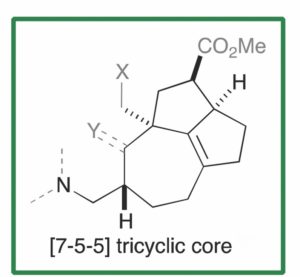
A recent OBC publication by Tohru Fukuyama and Satoshi Yokoshima of Nagoya University reports on the synthesis of a common structural core prevalent among the Daphniphyllum alkaloids. The common [7-5-5] tricyclic core features a quaternary carbon centre, two contiguous stereogenic centres and a tetrasubstituted C-C double bond.
The study began with the synthesis of the adjacent stereogenic centres, which was ultimately achieved through a Claisen-Ireland rearrangement with a 73% isolated yield (1 step) and dr = 6.3:1.
The challenging tetrasubstituted C-C double bond was tackled next. Limited procedures for the installation of the tetrasubstituted C-C alkene have been reported for the [7-5-5] tricyclic core. The highly congested nature of such substituted double bonds results in destabilizing eclipsing interactions, which are mirrored in the transition states leading to them. The tetrasubstituted C-C double bond of the [7-5-5] tricyclic core was therefore carried out using an E1cB-elimination of intermediate 28 to generate the a,b-unsaturated ketone 29 with a 78% isolated yield.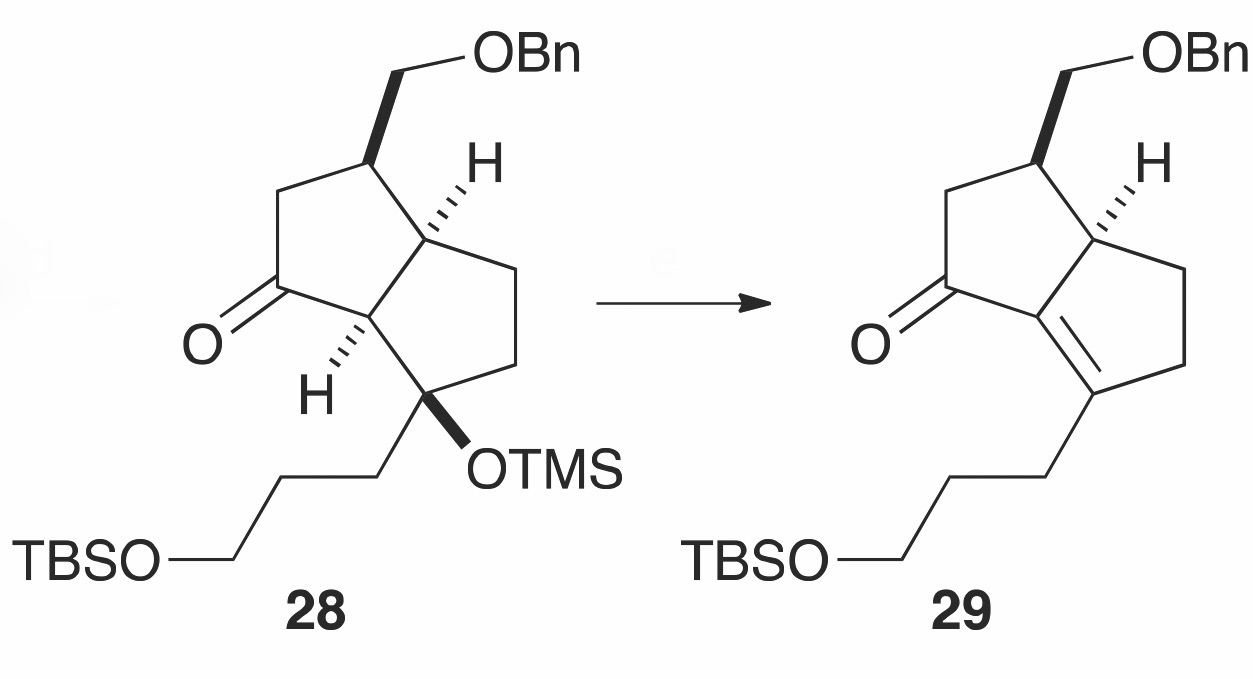
Extensive investigations revealed that the quaternary carbon centre could be accessed through a 2,3-Wittig rearrangement, which occurred stereoselectively on the less hindered face of the bicyclic intermediate. Quite efficiently, this transformation yielded intermediate 36 which contained a vinyl group that was to be used in the construction of the 7-membered ring. Final steps included a ring-closing metathesis and an intramolecular carbonyl ene reaction to complete the [7-5-5] ring system.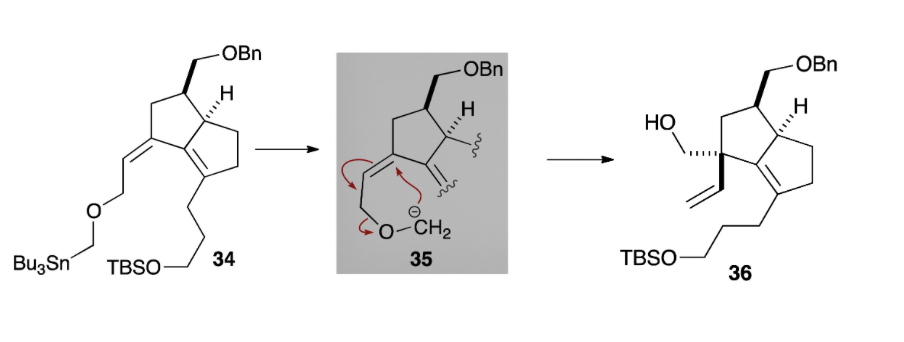
This creative study provides an excellent platform for the total synthesis of Daphniphyllum alkaloids that have the [7-5-5] tricyclic core.
To find out more see:
Synthesis of the [7-5-5] tricyclic core of Daphniphyllum alkaloids
Yusuke Kitabayashi, Tohru Fukuyama and Satoshi Yokoshima
DOI: 10.1039/C8OB00859K
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at the University of Toronto. Her research is centred on the synthesis of kinetically amphoteric building blocks which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.










