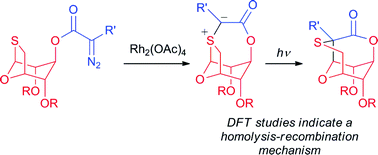
In this HOT paper Michael J. Porter and colleagues at University College London report the synthesis of the core structure of tagetitoxin using a novel photochemical 1,2-Stevens rearrangement. The method presented by Porter et al. uses the carbene-mediated ring expansion of a bicyclic monothioacetal, through the intermediacy of a sulfonium ylide.
To see all the experimental and mechanistic details download the paper today. It will be free to access for 4 weeks!
Inter- and intramolecular reactions of 1-deoxy-1-thio-1,6-anhydrosugars with α-diazoesters: synthesis of the tagetitoxin core by photochemical ylide rearrangement
Anne J. Price Mortimer, Julien R. H. Plet, Oluwafunsho A. Obasanjo, Nikolas Kaltsoyannis and Michael J. Porter
DOI: 10.1039/C2OB26308D