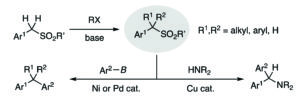
Compounds containing carbon-boron bonds are of high significance because they can be used as the precursors for various reactions such as 1,2-metallate rearrangements, deborylative nucleophilic addition reactions and the formation of carbon-oxygen and carbon-nitrogen bonds through hydrolysis and aminolysis respectively. Sulfones are useful for increasing the functionality of a molecule by alkylation and arylation (scheme 1), however till date there have been very few reports regarding the transformation of carbon-sulfonyl bonds to carbon-boron bonds. The transformation of sulfonyl groups to boryl groups under metal free organocatalysis is still a challenging task.
Scheme 1: Sequential functionalization of benzylic sulfones
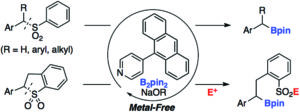
In their recent OBC publication, Prof Cathleen M. Crudden et.al. of Queen’s University, Ontario developed a beautiful protocol for the transition metal-free desulfonative borylation of benzyl sulfones using simple pyridine derivatives as catalysts (scheme 2). They reported the borylation of cyclic sulfones to afford functionalized sulfones and sulfonamides through a sulfinate intermediate which could be trapped with electrophiles. As they chose benzhydryl phenyl sulfone (1a) as a model substrate for their optimization, they reported the formation of the desired dibenzylic boronic (2a) ester along with diphenylmethane (3a) as a by-product. By using trifluorotoluene as a solvent instead of ethereal solvents, they optimized their conditions, resulting in an enhanced formation of 2a and supressed formation of 3a.
Scheme 2: Pyridine-promoted desulfonative borylation of benzyl sulfones
The reaction was well tolerated by a range of benzhydryl sulfones bearing both electron-neutral and electron-rich aryl groups with good yields. Even sterically hindered ortho-substituted aryl groups also afforded the desired products with lower yields. Unfortunately, benzhydryl sulfones bearing electron-withdrawing substituents such as trifluoromethyl, esters, cyano, allyl and iodide groups were not tolerated the transformation. Crudden et. al. executed control experiments in order to understand the reaction mechanism, finding that the sulfone bearing terminal olefin did not afford any product and also that TEMPO ((2,2,6,6-Tetramethylpiperidin-1-yl)oxyl) completely inhibited borylation. Based on these observations, they proposed a single electron transfer mechanism for borylation based on the work from Tuttle and Zhang and Jiao.
In conclusion, Crudden et al. succeeded in developing a desulfonylative borylation of alkyl sulfon es through 4-arylpyridine catalysis which yields synthetically useful benzylic boron compounds.
Read their full article now.
About the Blog Writer: A. Vamshi Krishna is currently pursuing a Ph.D. in organic chemistry with Prof. D. B. Ramachary at University of Hyderabad. His research mainly focuses on asymmetric supramolecular-organocatalysis, where he synthesises highly functionalized biologically active novel scaffolds with excellent selectivities and yields. His passion for scientific writing made him become a blog writer.