Polycyclic aromatic hydrocarbons (PAHs) containing heteroatoms have recently been grabbing the attention of scientists as they exhibit intriguing photophysical properties and can be widely used as building blocks for π-conjugated materials due to the effect of heteroatoms on the electronic properties of the system.
PAHs are often employed in OLEDs. Over the last few years, these PAHs have been synthesized in several different ways. Kawashima et.al synthesized different acene-like π-extended dibenzoborines such as compound B which show fascinating photophysical properties. More recently, Hatakeyama et.al have reported the synthesis of various PAHs that contain multiple 1,4-azaborine rings, such as compound C, which show excellent thermally activated delayed fluorescence. By investigating these types of molecules in more detail, their electronic properties can be improved to make better OLEDs.
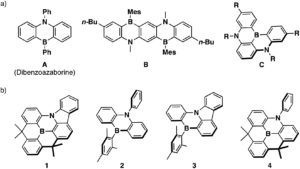
a) Representative examples of previously reported dibenzoazaborine-based pi-conjugated compounds and b) planarized B,N-phenylated dibenzoazaborine 1 together with reference compounds 2-4
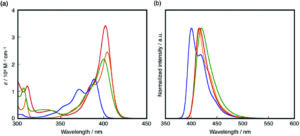
a) UV-vis absorption and b) fluorescence spectra of 1 (red), 2 (blue), 3 (green) and 4 (orange) in THF.
In their recent OBC publication, Professor Shigehiro Yamaguchi of the Institute of Transformative Bio- Molecules, Nagoya University et al. explored in detail the relation between the structure of materials and their electronic structures. They mainly focused on the correlation between the ring-fusion mode, where two cyclic rings fused in a planar manner, and the degree of structural constraint within the dibenzoazaborine skeleton. As constraining molecules into a planar fashion can extend π-conjugation and increase chemical stability, Shigehiro planarized the B-, N- phenyl groups in dibenzoazaborine to synthesize a new family of planarized triarylboranes with a carbazole substructure, confirming this structure by single-crystal X-ray diffraction analysis. They studied the photophysical properties of 1 and compared it with compunds 2-4. Compound 1 showed an intense absorption band at λabs= 402 nm unlike compounds 2-4 which showed λabs at 389 and 371, 400, 404 and 387 nm respectively. The fluorescence spectra of compound 1 showed an intense deep blue emission with a full width at half maximum (FWHM) of 27 nm which is due to its rigid structure. The optimized structure of 1 with respect to DFT calculations matched with the experimentally determined crystal structure. They report the time dependent DFT calculations which prove compound 1 to have the highest oscillator strength (f) amongst compounds 1-4, resulting in the largest molar absorption coefficient (ε). This clearly represents that it absorbs light strongly at the given wavelength. They also studied the electrochemical properties of 1-4 using cyclic voltammetry which resulted in one reversible reduction wave irrespective of the structural constraint showing that there are no electronic effects imposed on the structural constraint.
In conclusion, Shigehiro et al. succeeded in synthesizing structurally constrained, planarized dibenzoazaborines and investigating their photophysical and electronic effects.
Read their full article at https://rsc.li/2Xw8uoA.
Blog writer description: I am A. Vamshi Krishna, pursuing a PhD in organic chemistry with Prof. D. B. Ramachary at the University of Hyderabad. My research mainly focuses on asymmetric supramolecular-organocatalysis where we synthesise highly functionalized biologically active novel scaffolds with excellent selectivity and yields. I am passionate about scientific writing.










