According to statistics published by the World Health Organization (WHO), tuberculosis (TB) is globally one of the leading causes of death from a curable infectious disease. While antibiotics represent a major breakthrough for modern medicine, the spread of multi-drug resistant (MDR) bacterial strains, such as Mycobacterium tuberculosis, have become a major threat to healthcare.
Encouragingly, renewed efforts in antibiotic research have resulted in the identification of new leads, some of which are currently in clinical trials. Even in light of these promising efforts, Dr. Ehmke Pohl of Durham University and collaborators from the Cambridge Crystallographic Data Centre and Institut Pasteur de Lille are emphasizing the need to optimize existing TB treatments as well as develop an efficient means of identifying novel therapeutics.
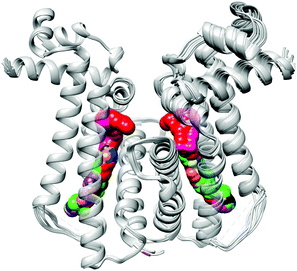 Structure-based drug discovery is an integral part of most industrial drug discovery programs and as a result, there is an ever-growing number of protein X-ray crystal structures available in databases such as the Protein Data Bank (PDB). Pohl and collaborators outline a robust and versatile strategy for an in silico screening protocol based on compounds in the ZINC database (a free resource of commercially available chemical compounds) and crystal structures in the PDB.
Structure-based drug discovery is an integral part of most industrial drug discovery programs and as a result, there is an ever-growing number of protein X-ray crystal structures available in databases such as the Protein Data Bank (PDB). Pohl and collaborators outline a robust and versatile strategy for an in silico screening protocol based on compounds in the ZINC database (a free resource of commercially available chemical compounds) and crystal structures in the PDB.
Their current OBC study focuses on the transcriptional regulator EthR which is involved in M. tuberculosis resistance. EthR has been shown to limit the efficacy of ethionamide-based drugs by downregulating the EthA enzyme involved in activation of ethionamide prodrugs. EthR has therefore been validated as a suitable target as its inhibition boosts ethionamide action.
Using tailored chemical and physicochemical descriptors (for example: compound volume) and a detailed knowledge of the EthR binding pocket, approximately 6 million compounds were evaluated for compatibility using KNIME pipeline software. 409 201 diverse compounds were identified for docking studies and surprisingly, only 6 compounds failed to produce feasible binding interactions. After a careful post-docking filter, 284 chemically diverse compounds were obtained and a visual analysis of all binding poses and ligand geometries in combination with computational analysis narrowed the screen down to 85 substrates. These in silico hits were then evaluated for their capability to bind to EthR using thermal protein stability studies which resulted in 20 new potential candidates for lead optimization with reasonable EC50 values.
Given the ever-growing number of high resolution crystal structures in the PDB, in silico screening approaches can be tailored to any well-characterized protein structure and utilized as an efficient tool for identifying new active molecules.
To find out more see:
New active leads for tuberculosis booster drugs by structure-based drug discovery
Natalie J. Tatum,
DOI:10.1039/C7OB00910K
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at the University of Toronto. Her research is centred on the synthesis of kinetically amphoteric building blocks which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.










