The reductive amination reaction between amines and carbonyls is a highly useful and versatile means of forming C-N bonds. Given the accessibility of starting materials and its modular nature, reductive aminations have found extensive application not only in organic synthesis but medicinal chemistry and the production of agro- and industrial chemicals.
Developing efficient and economical processes to access valuable materials is a priority in industry. One of the most fundamental ways of doing this is to adhere to the principle of atom economy which moves to minimize waste generated by a chemical reaction at the molecular level. While traditionally, chemists have focused on improving yield or minimizing the number of steps in a reaction sequence, atom economy aims to design reactions in which all atoms involved in a chemical process are present in the desired products.
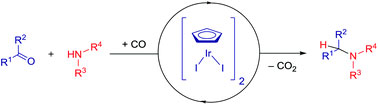
An international team of researchers have recently published a novel iridium-catalyzed reductive amination using carbon monoxide (CO) as an alternative reductant. This process does not require an external hydrogen source as the hydride is abstracted by the catalyst/carbon monoxide complex from the hemiaminal intermediate, forming an iridium-hydride species. Essentially, the hydride is derived internally (from the amine) as a result of the deoxygenative potential of carbon monoxide. The reaction is also tolerant to a number of functional groups that are incompatible with other commonly employed reducing reagents.
This is a very interesting twist on the reductive amination reaction for which external sources of hydrogen are often required. While it could be called atomic economic from this standpoint, the fact that carbon dioxide is a major by product of the reaction detracts from this claim and could be problematic on an industrial level. Regardless, this work is a significant first step and demonstrates the importance of optimizing the efficiency of well-established protocols in organic synthesis for large scale purposes.
To find out more see:
Reductive amination catalyzed by iridium complexes using carbon monoxide as a reducing agent
DOI:10.1039/C7OB01005B
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at the University of Toronto. Her research is centred on the synthesis of kinetically amphoteric building blocks which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.











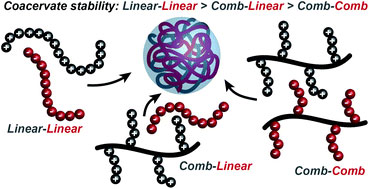 Under favourable conditions, the coalescence of coacervate droplets leads to a separation of a system into two liquid layers: a polymer-rich coacervate phase in equilibrium with a polymer poor supernatant. Coacervation is entropically driven and occurs through an initial electrostatic attraction between oppositely charged molecules followed by the release of counterions and rearrangement of water molecules. However, our understanding of factors that control self-assembly and stability at the molecular level remains limited.
Under favourable conditions, the coalescence of coacervate droplets leads to a separation of a system into two liquid layers: a polymer-rich coacervate phase in equilibrium with a polymer poor supernatant. Coacervation is entropically driven and occurs through an initial electrostatic attraction between oppositely charged molecules followed by the release of counterions and rearrangement of water molecules. However, our understanding of factors that control self-assembly and stability at the molecular level remains limited.