The rare phenomenon of enantioselectivity reversal, using a simple change in reaction temperature control, is presented in the latest HOT article published in OBC.
The ability to mimic nature’s stereochemical control in the production of complex molecules has been a longstanding challenge in chemistry. There are numerous strategies chemists have implemented to generate stereochemically complex structures, however, with the advent of asymmetric catalysis, highly stereoselective reactions can be achieved using chiral reagents and catalysts.
In a recent OBC publication by Prof. Kenso Soai of Tokyo University and researchers from Merck, one of the very few examples in which the enantioselective outcome of a reaction is controlled through temperature was presented. Gaining enantioselective control by changing simple reaction parameters has been an attractive and long sought after advancement within the field of asymmetric catalysis. While there are examples of enantioselective control using solvent, metals and additives, very few examples exist that use temperature alone.
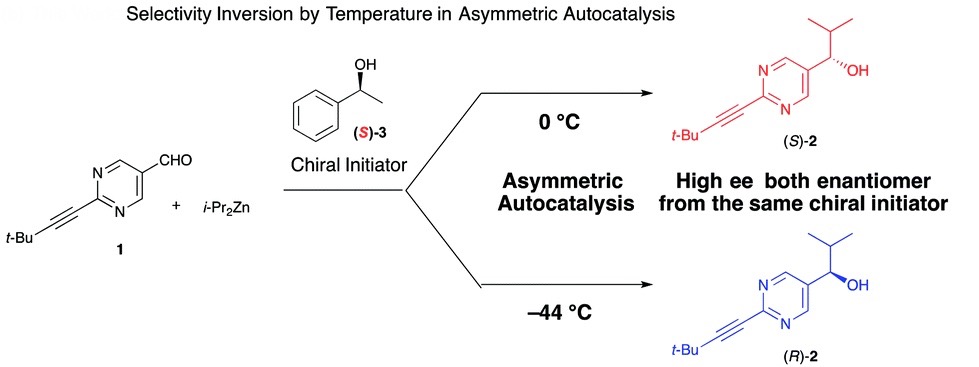
The study outlines the effect of temperature on the asymmetric autocatalysis of pyrimidal alkanol in the addition reaction of diisopropyl zinc to the pyrimidine-5-carbaldehyde. After reaction initiation using a chiral initiator, the product alkanol behaves as an asymmetric catalyst for its own formation and infers its chirality to the product in an autocatalytic cycle. When the reaction was performed at 0 ºC in the presence of (S)-1-phenyl-ethyl alcohol, as expected, (S)-pyrimidal alkanol was afforded in high enantiomeric excess. Interestingly, when the reaction was cooled to -44 ºC, the opposite enantioselectivity was observed though with a slightly lower enantiomeric excess of the desired alkanol.
The exact mechanism through which this reversal happens is still unclear however, it’s speculated that the relationship between temperature and the relative enthalpic vs. entropic contributions to free energy may play a part or the temperature dependent aggregation of zinc alkoxide may also be involved.
It’s important to remember that the temperature effect on reactions involving organozinc reagents is not always straight forward and may not always lead to the best outcome.
Regardless, this study provides interesting insight into temperature controlled enantioselectivity that may lead to a more detailed understanding of such processes and how they can be synthetically exploited.
To find out more see:
Unusual reversal of enantioselectivity in the asymmetric autocatalysis of pyrimidyl alkanol triggered by chiral aromatic alkanols and amines
Arimasa Matusmoto, Satoshi Fujiwara, Yui Hiyoshi,aKerstin Zawatzky, Alexey A. Makarov, Christopher J. Welch and Kenso Soai
DOI: 10.1039/C6OB02415G
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at The University of Toronto. Her research is centred on the synthesis of kinetically amphoteric molecules, which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.










