Antibiotic resistance has become a major clinical issue in recent decades and is one of the greatest health problems of our time. Tuberculosis (TB) has been present for thousands of years and according to WHO is globally, one of the leading causes of death from a curable infectious disease. The advent of the antibiotic era represented a major breakthrough for those suffering from TB however the spread of multi-drug resistant strains, which have propagated due to incorrect drug use, prescription errors or low patient compliance has become a major threat to disease control. In order to mitigate this, new drugs and diagnostic tools are desperately needed.
A collaborative study between Peter Woodruff of the University of Southern Maine and Benjamin Swarts of Central Michigan University has looked at in vivo nuclear imaging as a non-invasive means of diagnosing TB as well as disease monitoring and treatment response in real time. This means that vital information regarding disease progression that is complimentary to other forms of diagnosis can be quickly attained.
Current imaging methods rely on radiotracers to provide information on host inflammatory response to the infection however there can be some inaccuracy due to a lack of specificity in cellular uptake. An attractive alternative are radiolabeled analogues of antimycobacterial compounds which specifically label the desired bacteria and can provide in vivo imaging.
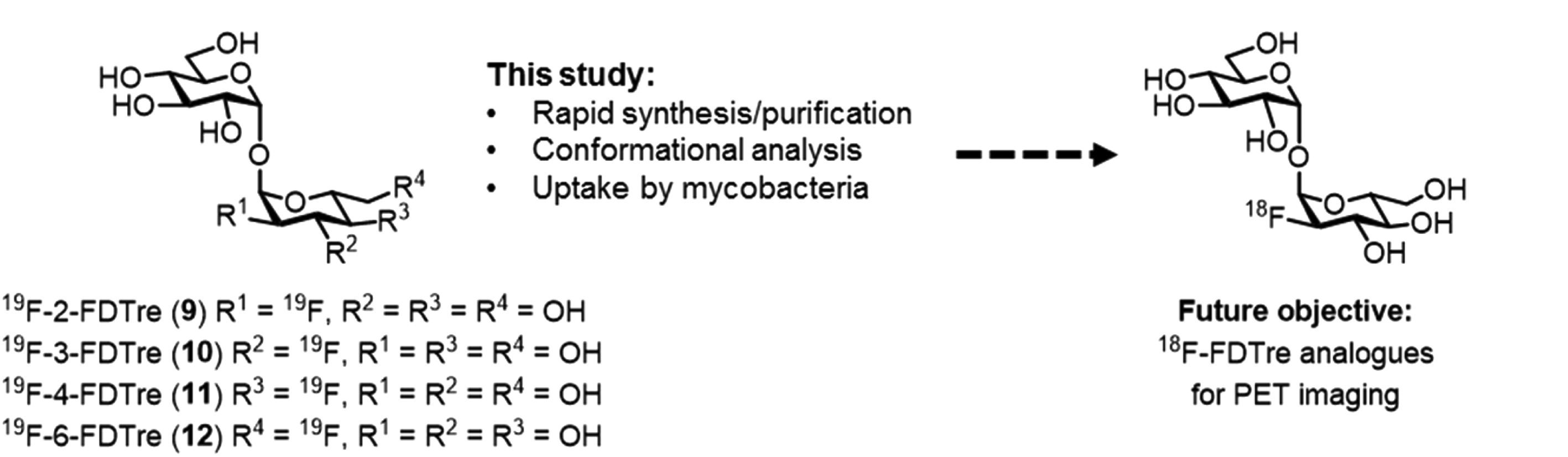
In the past, the disaccharide trehalose and its analogues have been applied to study metabolic mechanisms of mycobacteria and provide a means to design specifc probes for the mycobacteria induced infections. In this current publication, the researchers successfully developed a robust and concise synthetic route to access a number of fluorinated trehalose (FDTre) analogues using a rapid chemoenzymatic process followed by a simple purification by ion exchange chromatography. This drastic improvement compared to previously reported synthetic routes, which require lengthy reaction times and often result in low recovery, makes this new route highly desirable as well as compatible with timescale required for radiolabel synthesis. In addition, the authors demonstrated the ability of their FDTre analogues to be successfully recognized and taken up through trehalose transporter pathways in M. smegmatis and pathogenic mycobacteria, allowing for FDTre accumulation within cells.
Further investigations are still required to overcome remaining hurdles such as the cost associated with synthesis, assessment of uptake efficiencies at concentrations closer to in vivo radiotracer concentrations as well as evaluation of the speficity of the FDTre radioprobes. Regardless, the methodology established in this study provides an exceptional platform for the development of a new class of nuclear imaging probes to help treat multi-drug resistant TB and other mycobacterial infections.
To find out more see:
Deoxyfluoro-D-trehalose (FDTre) analogues as potential PET probes for imaging mycobacterial infection
Sarah R. Rundell, Zachary L. Wagar, Lisa M. Meints, Claire D. Olson, Mara K. O’Neill, Brent F. Piligian, Anne W. Poston, Robin J. Hood, Peter J. Woodruff and Benjamin M. Swarts
DOI: 10.1039/C6OB01734G
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at The University of Toronto. Her research is centred on the synthesis of kinetically amphoteric molecules, which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.