Target-based approaches to drug discovery have dominated pharmaceutical research since the early 1990s because they allow for increased screening capacity and rational drug design. Advances in combinatorial chemistry, high-throughput screening and gene expression profiling have been effective in the development of novel treatments for validated targets; however, a link between the downward trend in the number of new chemical entities reaching commercialization and the predominance of target-based drug discovery methods in the pharmaceutical industry has been suggested.
To address these issues, new technologies are constantly being developed and fragment-based drug design (FBDD) has emerged as a way to improve the quality and (most importantly) the efficiency of the drug discovery process. FBDD identifies the binding of low molecular weight ligands using techniques such as X-ray crystallography or NMR spectroscopy, and the binding information is then used to assemble potent lead compounds with drug-like properties.
In a recent publication, researchers at Astex Pharmaceuticals have noted that the success of FBDD often relies on the development of new synthetic methodologies for ligand elaboration. Despite the simplicity of fragment-like compounds, challenges lie in their design and synthesis as well as in developing the methodology to combine and grow fragments into high affinity leads. It is therefore important to identify compounds with attractive ‘fragment properties’ which are used as part of a selection process for adding new fragments into the Astex screening library. These include incorporation of diverse polar groups, multiple synthetically accessible positions for fragment growth in three dimensions and synthetic tractability among others.
Dihydroisoquinolone and some of its derivatives were identified in this study as ideal fragment-like compounds for the development of a FBDD-friendly synthetic methodology.
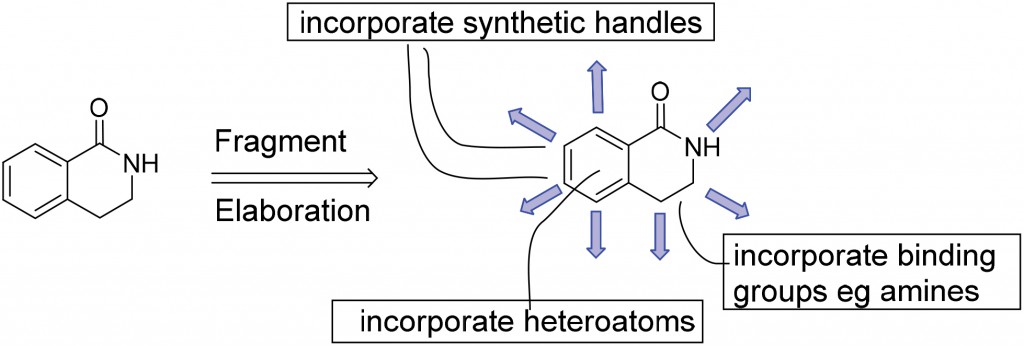 |
As proof of concept, the previously reported synthesis of dihydroisoquinolone-based compounds using Rh(III)-catalyzed C-H bond activation1 was modified to incorporate additional potential binding groups as well as synthetic handles for efficient fragment-to-lead elaboration.
Their results illustrate an excellent example of how the application of FBDD-friendly synthetic methodology can be used to expand upon published methodologies to increase their utility in developing useful templates for fragment-based drug discovery. Although inventive ideas, such as this work, are making small contributions in pharmaceutical research, there is a need for more organic chemists to engage in these synthetic challenges if drug discovery techniques are to be fruitful in the long-run.
1 N. Guimond et al. J. Am. Chem. Soc., 2011, 133(16), 6449–6457
To find out more see:
Design and synthesis of dihydroisoquinolones for fragment-based drug discovery (FBDD)
Nick Palmer, Torren M. Peakman, David Norton and David C. Rees
DOI: 10.1039/C5OB02461G
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at The University of Toronto. Her research is centred on the synthesis of kinetically amphoteric molecules, which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.










