Research efforts carried out by Professor Timothy Donohoe of Oxford University have been focussed on connecting new methodologies in organic synthesis and catalysis with impactful applications to the fields of medicinal chemistry and natural product synthesis.
One of the group’s most recent endeavors includes the development of a generalized strategy to access highly functionalized and diverse isoquinoline cores without the use of expensive and highly-specialized starting materials.
The isoquinoline motif and its derivatives are ubiquitous in a number of natural products, pharmaceutical agents, and chiral catalyst ligands. However, classical syntheses are often centered on electrophilic aromatic substitution of electron-rich systems, resulting in limited chemical diversity in accessible products. New routes are still highly desirable and a resurgence in synthetic efforts has resulted in a number of notable contributions using modern synthetic methodology.
In 2012, Prof. Donohoe and coworkers reported a sequential palladium-catalyzed α-arylation of enolates and cyclization to access isoquinolines based on chemistry originally and independently reported by Buckwald, Hartwig and Miura in 1997. Though a powerful reaction, it remained underutilized in the assembly of complex aromatic compounds. Using clever reaction engineering, Donohoe and coworkers envisioned synthesizing a psuedo-1,5-dicarbonyl accessible through α-arylation of enolizable ketones with aryl halides possessing a protected aldehyde or ketone in the ortho-position. In addition, trapping with reactive electrophiles resulted in functionalization at the C4 position. This methodology can be carried out in one pot, tolerates a wide range of substituents and most notably, provides a route to synthetically challenging electron-deficient isoquinoline scaffolds.
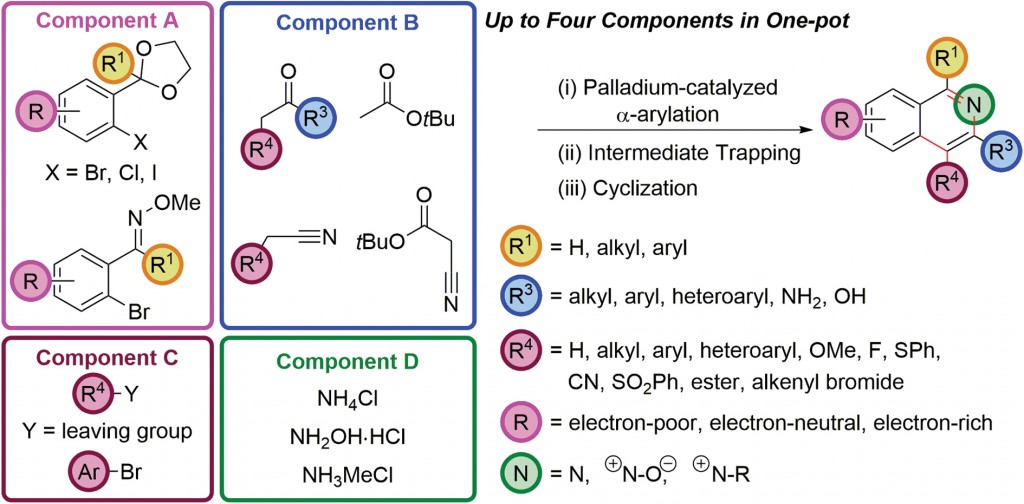 |
Their current study presents significant extensions of this earlier work and further demonstrates the innovation possible through transition metal catalysis in enabling the construction of complex architectures in interesting ways. The three- and four-component coupling procedures involve multiple bond formations in one pot from largely commercially available starting materials. Reaction versatility is demonstrated through the use of ketone, ester or nitrile enolates as well as electron-rich, electron-deficient or even sterically hindered aryl halides and in situ functionalization of intermediates to directly access a number of highly functionalized isoquinoline based compounds.
In addition to rejuvenating interesting and underexplored chemistry, Prof. Donohoe and coworkers have appreciably impacted the areas of natural product synthesis and medicinal chemistry through their innovative and streamlined synthesis of isoquinoline-based compounds and it will be interesting to see where their future endeavours will lead.
To find out more see:
Palladium-catalyzed enolate arylation as a key C–C bond-forming reaction for the synthesis of isoquinolines
Ben S. Pilgrim, Alice E. Gatland, Carlos H. A. Esteves, Charlie T. McTernan, Geraint R. Jones, Matthew R. Tatton, Panayiotis A. Procopiou and Timothy J. Donohoe
DOI: 10.1039/C5OB02320C
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at The University of Toronto. Her research is centred on the synthesis of kinetically amphoteric molecules, which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.










