Posted on behalf of Steve Moore, web writer for Organic & Biomolecular Chemistry
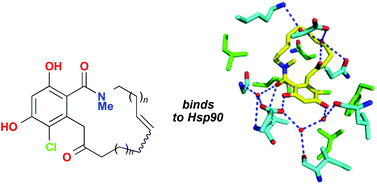
In their search for new inhibitors of Hsp90, scientists from the University of Nottingham and the University of Sussex have synthesised a series of macrolactam radicicol analogues. A new synthetic route to N-methylated resorcylic acid macrolactams is described which permits convenient variation of ring size. Macrolactam binding to Hsp90 was demonstrated by isothermal calorimetry and conformational changes were observed in co-crystallization experiments with yeast Hsp90.
Synthesis of macrolactam analogues of radicicol and their binding to heat shock protein Hsp90
Bridie L. Dutton, Russell R. A. Kitson, Sarah Parry-Morris, S. Mark Roe, Chrisostomos Prodromou and Christopher J. Moody
Org. Biomol. Chem., 2014, DOI: 10.1039/C3OB42211A, Paper
Free to access until: 24th March Download PDF | Download HTML










