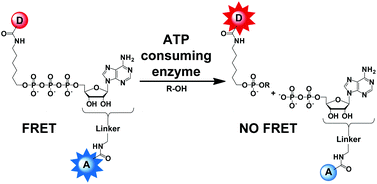
This HOT article describes the synthesis of ATP analogues labelled with a fluorescence donor and a fluorescence acceptor, suitable for Förster Resonance Energy Transfer. Excitation promotes energy transfer from the fluorescence donor to the fluorescence acceptor on the same molecule. Cleavage of the ATP analogue prevents energy transfer via an intramolecular pathway, resulting in a large change in fluorescence. Synthetic routes to seven new doubly labelled ATP analogues are reported and the fluorescence properties of these molecules are described using snake venom phosphodiesterase as a model enzyme.
Synthesis and fluorescence characteristics of ATP-based FRET probes
Norman Hardt, Stephan M. Hacker and Andreas Marx
Org. Biomol. Chem., 2013, DOI: 10.1039/C3OB41751D










