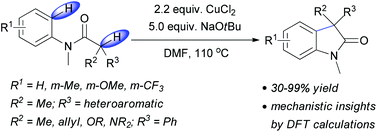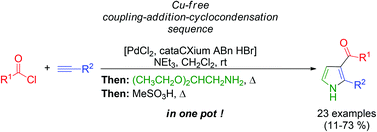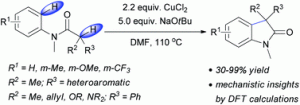
In this HOT paper, Peter Kündig’s research team have developed a new mode of copper-mediated oxidative coupling allowing access to racemic (aza)oxindole structure in an efficient and atom-economic fashion.
Oxindoles are useful structures in organic chemistry as they are found ubiquitously in nature. Molecules bearing this motif have a range of interesting biological properties and new methods of their construction are always useful to the organic chemist.
Peter Kündig and his team at the University of Geneva have been looking into the asymmetric synthesis of these structures, employing palladium-catalysed intramolecular α-arylations of amide substrates.[1] During these studies, they discovered a novel and efficient route to access racemic 3,3,-disubstituted oxindoles which employed a copper-mediated radical reaction.[2] This reaction has also been applied to the racemic synthesis of aza-oxindoles.[3]
In this excellent account from Prof. Kündig, this methodology has been further explored. The substrate scope has been expanded leading to a range of oxindoles bearing a variety of aromatic, hetero- aromatic, allyl and heteroatom substituents in the 3-position. The reaction was also successfully performed on a large scale, with excellent yield and regioselectivity. In addition to this, some nifty DFT and computational studies confirm the proposed mechanism and regioselectivity.
Read the full article here.
Copper(II) chloride mediated (aza)oxindole synthesis by oxidative coupling of Csp2–H and Csp3–H centers: substrate scope and DFT study
Chandan Dey, Evgeny Larionov and E. Peter Kündig
Org. Biomol. Chem., 2013, DOI: 10.1039/c3ob41254g
[1] E. P. Kündig, T. M. Seidel, Y. X. Jia and G. Bernardinelli,
Angew. Chem., Int. Ed., 2007,
46, 8484; Y. X. Jia, J. M. Hillgren, E. L. Watson, S. P. Marsden and E. P. Kündig,
Chem. Commun., 2008, 4040.
[2] Y. X. Jia and E. P. Kündig, Angew. Chem., Int. Ed., 2009, 48, 1636.
[3] C. Dey and E. P. Kündig, Chem. Commun., 2012, 48, 3064.