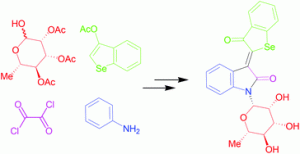
Synthetic indirubin derivatives can selectively inhibit cyclin-dependent kinases, stalling cancer growth and proliferation.
Peter Langer and co-workers hypothesised that selenoindirubins could have similar anti-proliferative effects. Selenoindirubins are scarcely reported in the literature, with only three examples known. This HOT article details an optimized synthesis for a series of selenoindirubins, alongside the first report of spectroscopic data for this class of compounds. A glycosyl moiety increased the pharmacological activity of indirubin derivatives and was incorporated into the selenoindirubin series to improve their solubility in DMSO and water. Members of this series had an anti-proliferative effect in lung cancer cells. Apoptosis was enhanced by combination treatment with the death ligand TRAIL, suggesting that selenoindirubins may have potential applications as anti-tumour agents.
Synthesis and antiproliferative activity of selenoindirubins and selenoindirubin-N-glycosides
Friedrich Erben, Dennis Kleeblatt, Marcel Sonneck, Martin Hein, Holger Feist, Thomas Fahrenwaldt, Christine Fischer, Abdul Matin, Jamshed Iqbal, Michael Plötz, Jürgen Eberle and Peter Langer
DOI: 10.1039/c3ob40603b
Free to access for 4 weeks