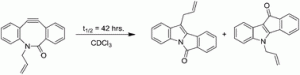
In this HOT paper, John Pezacki and co-workers report novel rearrangement and addition reactions of biarylazacyclooctynone (BARAC) leading to tetracyclic products. This behaviour may limit the practical applications of azacyclooctynones as bioorthogonal probes for biological systems.
Bioorthogonal reactions, i.e. reactions which can occur inside of a living system without interfering with native chemical processes, allow for the study of molecules such as proteins and lipids in vivo and in real time. The 1,3-dipolar cycloaddition between azides and octynes is an example of such a reaction. This copper-free variant of the Huisgen cycloaddition (better known as the click reaction) has been applied within cultured cells, live zebrafish and mice.
John Paul Pezacki and his research group at National Research Council Canada have been looking into these reactions and their application in biological probes and sensors. During their studies into the cycloaddition of azacyclooctynones such as BARAC (biarylazacyclooctynone), they noticed some interesting and unexpected results.
These molecules are able to undergo novel intramolecular cyclisation reactions which lead to the formation of tetracyclic products. Pezacki has performed some neat kinetics studies and computer-modelling, which have revealed that this rearrangement is accelerated by the presence of acid and that the linker side-chain also influences the rate of rearrangement.
This elegant, but rather unhelpful, reaction may limit the effectiveness of these molecules in biological systems, and this fascinating study illustrates how challenging it is to design effective bioorthogonal reactions.
Rearrangements and addition reactions of biarylazacyclooctynones and the implications to copper-free click chemistry
Mariya Chigrinova, Craig S. McKay, Louis-Philippe B. Beaulieu, Konstantin A. Udachin, André M. Beauchemin and John Paul Pezacki.
DOI: 10.1039/C3OB40683K
Free to access for 4 weeks