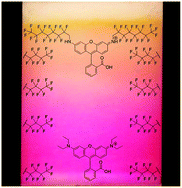
In this HOT article Bräse and co-workers report the synthesis of eight new rhodamine fluorophores with fluorous ‘ponytails’, dubbed rhodamine F dyes. Rhodamine derivatives are widely used as fluorescent labels for biomolecules and characteristically have high absorption coefficients and high absorption and emission maxima in the visible region combined with high chemical and photostability.
The ‘ponytails’ are fluorous alkyl residues that are deliberately not cleaved during synthesis. They facilitate fluorous solid-phase extraction, utilising the strong, specific nature of fluorine-fluorine interactions. During this purification process a fluorous solid phase is used to bind fluorous molecules and separate them from non-fluorous compounds.
The fluorine content of the rhodamine F dyes is below 50% of the total molecular weight, preserving solubility in organic solvents. Conjugation with a peptide mediates cellular uptake may have potential applications in live cell imaging; clear, bright images were obtained in human cervix carcinoma cells incubated with rhodamine F dyes at concentrations as low as 1 µM.
Rhodamine F: a novel class of fluorous ponytailed dyes for bioconjugation
Dominik K. Kölmel, Birgit Rudat, Delia M. Braun, Christin Bednarek, Ute Schepers and Stefan Bräse
DOI: 10.1039/c3ob40267c
Free to access for 4 weeks
Published on behalf of Steve Moore, Organic & Biomolecular Chemistry web science writer.