In this HOT article, Julius Rebek Jr presents a fascinating insight into the design of molecules which can detect and destroy organophosphorus compounds.
Organophosphorus (OP) compounds are primarily used as pesticides, and can be a useful alternative to more traditional chlorinated hydrocarbon-based pesticides. However, due to OP compounds’ ability to inhibit acetylcholine esterase (AChE), they can be extremely toxic to humans and they have therefore found use as nerve agents and chemical weapons. For these reasons, their toxicity is an area of intense research focus and new methods for their detection and treatment are always welcome.
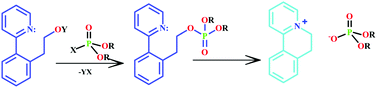
Rebek and his research group at The Scripps Research Institute have been looking into molecular sensors for OPs for several years, and this paper highlights some of the significant developments in the field. Their interest in the area was sparked by a carefully crafted pyridine structure discovered by Swager, which upon reaction with an OP undergoes a subsequent cyclisation reaction to create a fluorescent dye. This is a useful premise for the sensing of OPs. The Rebek group have expanded upon this premise and developed a series of molecular sensors that employ similar mechanisms. They have used their established expertise in cavitand chemistry to develop specially-tailored vase-shaped molecules, which are able to fold around OP-based nerve agents and isolate them from the surrounding medium. This paper provides a whistle-stop tour of an exciting and important area of organic chemistry.
Chemical approaches for detection and destruction of nerve agents
Dariush Ajami and Julius Rebek, Jr.
DOI: 10.1039/c3ob40324f
Free to access for 4 weeks