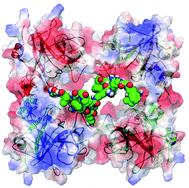
 In this HOT article Carsten Schmuck and co-workers report the synthesis and evaluation of a series of four-armed peptide ligands that can inhibit β-tryptase. These tetravalent ligands are made up of 2 sets of arms incorporating the artificial arginine analogue guanidiniocarbonyl pyrrole cation and can inhibit β-tryptase in a reversible and non-competitive way by binding to anionic hotspots on the surface of the enzyme.
In this HOT article Carsten Schmuck and co-workers report the synthesis and evaluation of a series of four-armed peptide ligands that can inhibit β-tryptase. These tetravalent ligands are made up of 2 sets of arms incorporating the artificial arginine analogue guanidiniocarbonyl pyrrole cation and can inhibit β-tryptase in a reversible and non-competitive way by binding to anionic hotspots on the surface of the enzyme.
Read the complete study for free for 4 weeks!
A new approach to inhibit human β-tryptase by protein surface binding of four-armed peptide ligands with two different sets of arms
Qian-Qian Jiang, Lina Bartsch, Wilhelm Sicking, Peter R. Wich, Dominik Heider, Daniel Hoffmann and Carsten Schmuck
DOI: 10.1039/C3OB27302D











