Dirk Menche and co-workers report an efficient procedure for the concise synthesis of highly elaborate polyenes. They have showcased their novel hetero-bis-metallated alkene reagent in the construction of a small library of etnangien side-chain analogues. This has allowed them to establish important structure-activity information about this family of biologically-significant natural products.
| Modular synthesis of polyene side chain analogues of the potent macrolide antibiotic etnangien by a flexible coupling strategy based on hetero-bis-metallated alkenes Mario Altendorfer, Aruna Raja, Florenz Sasse, Herbert Irschik and Dirk Menche DOI: 10.1039/C2OB26906F |
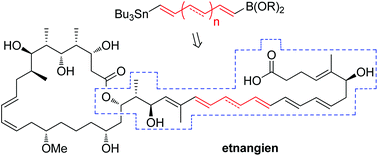
Dirk Menche and his group at the University of Heidelberg have been investigating the polyketide etnangien, a potent antibiotic that inhibits RNA-polymerase. The molecule is named for Mt. Etna, the soil of which yielded the producing strain of myxobacteria Sorangium cellulosum.
A macrolactone with an extensive polyene sidechain and boasting 12 stereogenic centres, this molecule is no straightforward synthetic target. It is also extremely unstable which adds to the challenge.
Menche and co-workers developed the first total synthesis of this intriguing molecule a few years ago, and were able to elucidate the complex stereochemistry. They also found that its methyl ester analogue is considerably more stable, and retains much of the parent compound’s potency. Since then, they have been investigating the biological properties of etnangien and related structural analogues.
Previous studies have shown that the macrocycle alone bears little activity, suggesting that the polyene side-chain is at least partially responsible for the biological interaction.
In their latest HOT PAPER, Menche et al. set out to probe this idea. They have developed a novel bis-metallated alkene reagent, bearing a tributyltin moiety at one end, and a pinacolborane at the other. A modular late-stage Stille/Suzuki cross-coupling sequence allowed for construction of the etnangien polyene sidechain. This approach was also applied to the manufacture of a small range of related analogues, and these were all tested for biological activity.
They found that all of the sidechains exhibit no antibiotic activity, confirming the importance of the macrocycle for biological potency. This information suggests that both macrocycle and sidechain are essential for full antibacterial activity. The group will be further exploring these results, and applying them to the future design of novel potent but more stable inhibitors of RNA-polymerase.
Published on behalf of Annabella Newton, Organic & Biomolecular Chemistry web science writer. Annabella Newton is a postdoctoral researcher based in Melbourne, Australia.










