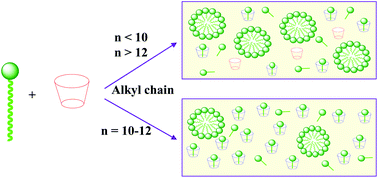
In this HOT paper Cepeda and co-workers explore the solution phase behaviour of β-cyclodextrin/alkyltrimethylammonium bromide mixtures, varying the surfactant alkyl chain length from hexyl (C6) to octadecyl (C18).
Cepeda et al. employ the solvolysis of methoxybenzenesulfonyl chloride (MBSC) as a UV ‘handle’ to monitor the interaction between β-cyclodextrin and surfactant guests. In the presence of aggregated cationic surfactants, MBSC hydrolysis is hindered, owing to the reduced polarity of the micelle interior. MBSC solvolysis can be used to monitor the integrity of surfactant micelles and to calculate the concentration of unbound cyclodextrin.
In the presence of aggregated surfactants Cepeda et al. detect a significant concentration of ‘free’ β-cyclodextrin; the highest of which with decyl (C10) and dodecyl (C12) surfactants. This reflects the balance between the hydrophobic effect (smaller surfactant chains displace less water from the cyclodextrin cavity) and surfactant self-assembly (longer surfactant chains favour micelle formation). The new model created to describe these interactions will aid the further development of commercial applications for these systems.
Competition between surfactant micellization and complexation by cyclodextrin
M. Cepeda, R. Daviña, L. García-Río, M. Parajó, P. Rodríguez-Dafonte and M. Pessêgo
DOI: 10.1039/C2OB26318A
Published on behalf of Steve Moore, Organic & Biomolecular Chemistry web science writer.










