Researchers from Taiwan have developed an efficient organocatalytic enantioselective synthesis of spironitrocyclopropanes.
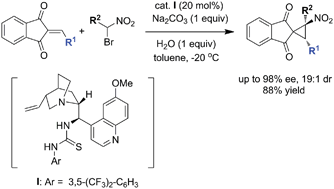
Lin et al. report reaction conditions which allow almost perfect diastereocontrol as well as outstanding enantiocontrol. As nitrocyclopropanes are found in various biologically active natural products and important precursors of bio-relevant cyclopropylamines this new method fills a real synthetic need.
Want to know more? Read the communication for free now! And it will remain free for the next 4 weeks.
An efficient organocatalytic enantioselective synthesis of spironitrocyclopropanes
Utpal Das, Yi-Ling Tsai and Wenwei Lin
DOI: 10.1039/C2OB26943K










