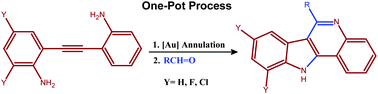
In this HOT paper Antonio Arcadi, Università degli Studi di L’Aquila, and colleagues present a newly developed one-pot gold-catalysed approach to the assembly of 11H-indolo[3,2-c]quinolines from readily available unprotected 2-[2-(2-aminophenyl)ethynyl]anilines and aldehydes.
Arcadi et al. use the clever idea of first using a fast regio-selective intramolecular reaction, followed by a slower intermolecular condensation and subsequent cyclisation and final hydrogen transfer nicely provides a fast, synthetically efficient and elegant access to indolo[3,2-c]quinolines. With its broad scope, mild conditions and high regioselectivity this could be a valuable alternative to protocols previously described in the literature.
Find out more by downloading this paper today. It’s free to access for 4 weeks so grab it whilst you can.
An alternative one-pot gold-catalyzed approach to the assembly of 11H-indolo[3,2-c]quinolines
Giorgio Abbiati, Antonio Arcadi, Marco Chiarini, Fabio Marinelli, Emanuela Pietropaolo and Elisabetta Rossi
DOI: 10.1039/C2OB26380G











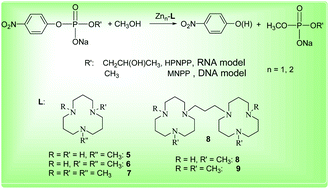 This manuscript from Zhong-Lin Lu and co-workers presents the preparation of a series of mononuclear and dinuclear zinc(II) complexes with ligands bearing different numbers of N-methyl groups, and includes a thorough kinetic study towards the hydrolytic cleavage of RNA and DNA model substrates.
This manuscript from Zhong-Lin Lu and co-workers presents the preparation of a series of mononuclear and dinuclear zinc(II) complexes with ligands bearing different numbers of N-methyl groups, and includes a thorough kinetic study towards the hydrolytic cleavage of RNA and DNA model substrates.