Biscarbazoles are a class of carbazole alkaloids in which two carbazole moieties are connected by different linkages. Oxydimurrayafoline is a special biscarbazole connecting two carbazole units via a benzylic ether linkage at the 3-position.
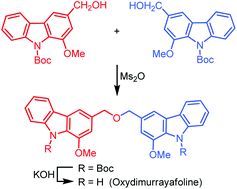
Knölker et al. use a palladium catalysis approach to produce the key intermediate mukonine in 2 steps before going on to make oxydimurrayafoline in a total of 6 steps with an overall yield of 43%.
This Communication will be FREE to access for the next 4 weeks, so have a look at the details of the study today.
First total synthesis of the biscarbazole alkaloid oxydimurrayafoline
Carsten Börger, Micha P. Krahl, Margit Gruner, Olga Kataeva and Hans-Joachim Knölker
DOI: 10.1039/C2OB25842K










