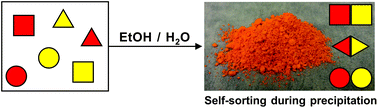
A judicious choice of precipitation conditions can lead to self-sorting of equilibrating mixtures of aromatic aldehydes and substituted anilines into a handful of imine products, say researchers from the US.
You might expect to get quite a few different products but because the least soluble imine precipitates, the mixture re-equilibrates to generate more of that compound. Eventually, all precursors to the least soluble imine are exhausted and the mixture is reduced in complexity. Only the most and the least soluble imines are produced without any of the “in between” species.
A mixture of as many as nine imines could be self-sorted into just three compounds, they say.
Precipitation-driven self-sorting of imines
Rio Carlo Lirag , Karolina Osowska and Ognjen Š. Miljanić
DOI: 10.1039/C2OB25736J