The first ever Organic & Biomolecular Chemistry International Symposium starts next week!
| Monday 16th April 2012 – Shanghai Institute of Organic Chemistry, Shanghai, China
Wednesday 18th April 2012 – Lanzhou University, Lanzhou, China Friday 20th April 2012 – Peking University, Beijing, China |
Each of these one-day meetings feature a selection of lectures covering organic and bioorganic chemistry by some of the world’s leading international scientists, including:
• Professor Jeffrey Bode (ETH Zürich, Switzerland): Amide Bond Formation: Taking N-O for an Answer
• Professor Dirk Trauner (Ludwig Maximilian University Munich, Germany): Exploring the Limits of Biomimetic Synthesis
• Professor Andrei Yudin (University of Toronto, Canada): Amphoteric Molecules – A Powerful Platform for Reaction Discovery
Plus many leading scientists from each of the host organisations!
The symposium is ABSOULUTELY FREE and NO REGISTRATION is needed so you can just turn up on the day, making it perfect for students as well!
For more information please see the symposium website.
We look forward to seeing you there!














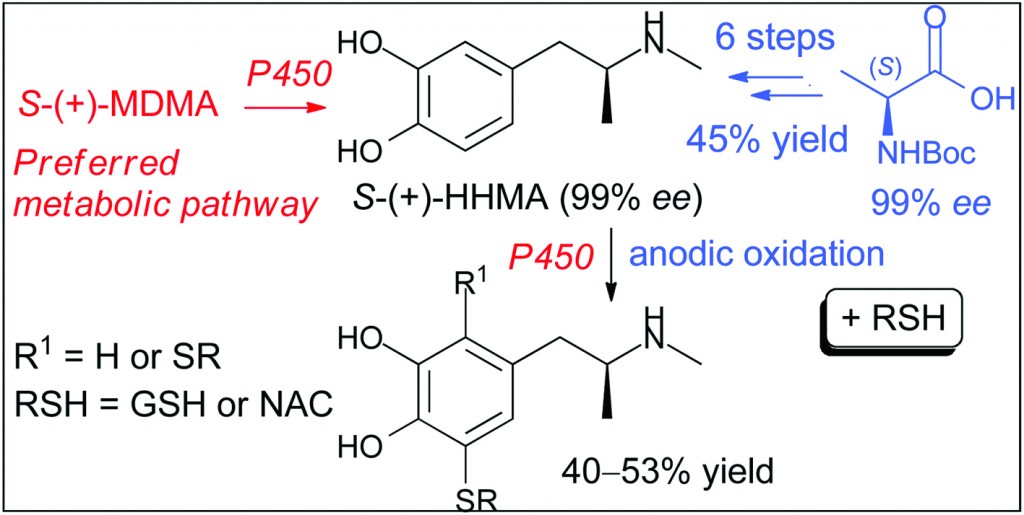 MDMA, also known as “ecstasy”, is a psychoactive drug with selective neurotoxic potential toward brain serotonin neurons. Despite intensive research, the precise mechanism by which MDMA selectively damages brain neurons in most species remains unknown. One hypothesis is that MDMA neurotoxicity may at least partially be a consequence of its metabolism. In particular, O-demethylenated MDMA metabolites such as N-methyl-α-methyldopamine (HHMA) have been postulated to serve as precursors for toxic catechol–thioether conjugates.
MDMA, also known as “ecstasy”, is a psychoactive drug with selective neurotoxic potential toward brain serotonin neurons. Despite intensive research, the precise mechanism by which MDMA selectively damages brain neurons in most species remains unknown. One hypothesis is that MDMA neurotoxicity may at least partially be a consequence of its metabolism. In particular, O-demethylenated MDMA metabolites such as N-methyl-α-methyldopamine (HHMA) have been postulated to serve as precursors for toxic catechol–thioether conjugates.