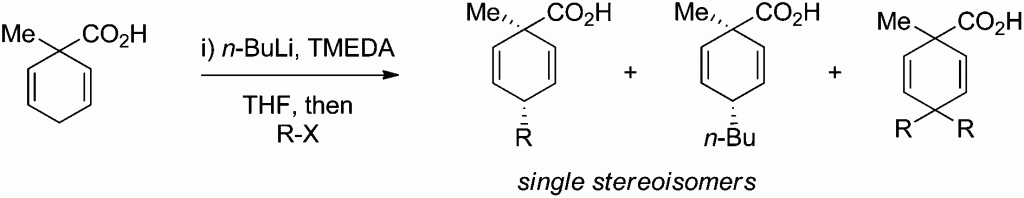
In this HOT paper Mark C. Elliott and colleagues at Cardiff University present an investigation on the deprotonation and alkylation of 1-methylcyclohexa-2,5-diene-1-carboxylic acid under a range of conditions. The culmination of the investigation shows that deprotonation of 1-methylcyclohexa-2,5-diene-1-carboxylic acid with n-BuLi in the presence of TMEDA, followed by alkylation, offers a direct and highly diastereoselective route to the corresponding 4-substituted products in which the alkyl group introduced is transto the carboxylic acid.
Mark C. Elliott et al. comment that this result “complements previous findings of H. van Bekkum in which a 4-substituted benzoic acid is reductively alkylated and where, when any selectivity is observed, the alkyl group and the carboxylic acid are preferentially cis.”
Click here for the complete study…..
This HOT article will be FREE TO ACCESS for the next 4 weeks!
Diastereoselective alkylation reactions of 1-methylcyclohexa-2,5-diene-1-carboxylic acid
Nicholas J. Bennett, Mark C. Elliott, Natalie L. Hewitt, Benson M. Kariuki, Clare A. Morton, Steven A. Raw and Simone Tomasi
Org. Biomol. Chem., 2012
DOI: 10.1039/C2OB25211B










