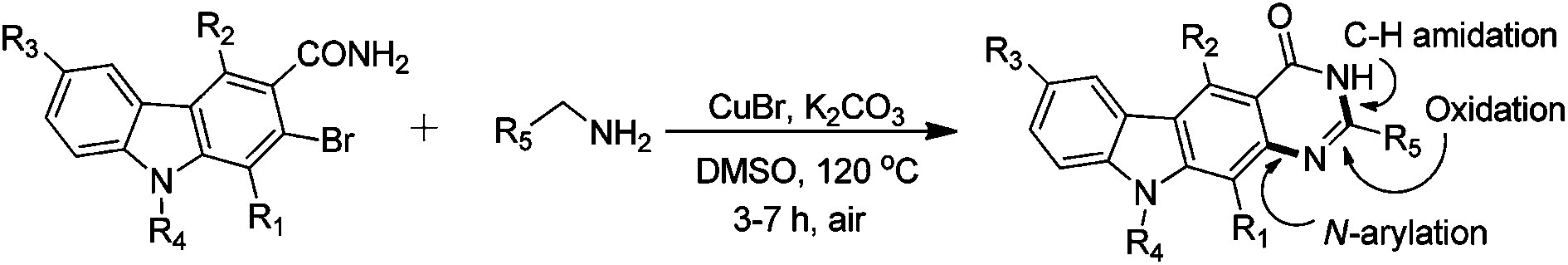Rajagopal Nagarajan and colleagues at University of Hyderabad have synthesized a series of pyrimido[4,5-b]-carbazolone derivatives using cascade Ullmann N-arylation and aerobic oxidative C–H amidation reactions that allow the assembly of readily accessible building blocks into diverse (heteroarylannulated) pyrimido[4,5-b]carbazolones with the aid of CuBr as a catalyst without any additives or ligands.
An attractive feature of this synthetic approach is that it not only provides a new approach for constructing pyrimido[b]carbazoles but also offers an efficient method for preparation of synthetically and medicinally important hetero-arylated carbazoles.
For all the details of their investigation simply CLICK HERE. This HOT article is FREE to access for 4 weeks!
What are your thoughts on this paper? Why not share them by leaving a comment below.
Copper-mediated domino synthesis of pyrimido[4,5-b]carbazolones via Ullmann N-arylation and aerobic oxidative C–H amidation
Devanga K. Sreenivas, Nagarajan Ramkumar and Rajagopal Nagarajan
Org. Biomol. Chem., 2012,
DOI: 10.1039/C2OB07179G