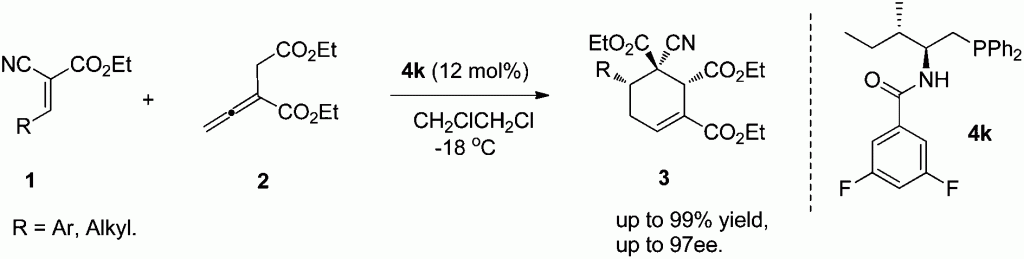
Gang Zhaoet al. from University of Science and Technology of China present highly diastereoselective and enantioselective [4 + 2] cycloadditions between allenoates and dual activated olefins catalyzed by bifunctional N-acyl aminophosphine catalysts.
To demonstrate the potential of the N-acyl aminophosphine catalysts in asymmetric phosphine mediated reactions, Gang Zhao et al. have prepared a range of synthetically valuable cyclohexene structures based on α-substituted buta-2,3-dienoates, which bear three contiguous chiral centers with good to excellent diastereoselectivities and enantioselectivities.
Take a look and let us know what you think!
This HOT OBC article will be free to access for the next 4 weeks.
Highly enantioselective [4 + 2] cycloadditions of allenoates and dual activated olefins catalyzed by N-acyl aminophosphines
Hua Xiao, Zhuo Chai, Dongdong Cao, Hongyu Wang, Jinghao Chen and Gang Zhao
Org. Biomol. Chem., 2012,
DOI: 10.1039/C2OB25295C