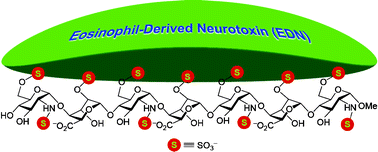
Gold-mediated bifunctional modification of oligosaccharides via a three-component coupling reaction
Karen Ka-Yan Kung, Gai-Li Li, Lan Zou, Hiu-Chi Chong, Yun-Chung Leung, Ka-Hing Wong, Vanessa Kar-Yan Lo, Chi-Ming Che and Man-Kin Wong
DOI: 10.1039/C1OB06429K

Hydroxyapatite-supported Cu(I)-catalysed cyanation of styrenyl bromides with K4[Fe(CN)6]: an easy access to cinnamonitriles
Debasree Saha, Laksmikanta Adak, M. Mukherjee and Brindaban C. Ranu
DOI: 10.1039/C1OB06467C
View the rest of the issue including a review on squaraine dyes in photodynamic therapy











 In this hot article
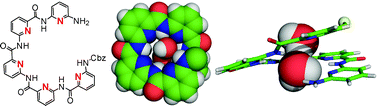
In this hot article 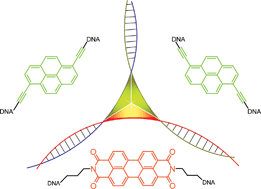 The naturally occurring 3-way junction formed at the branch point of three DNA or RNA strands is a useful structure for organising functional molecules. In this hot paper Markus Probst et al. have used the three-way junction to assemble non-nucleosidic alkynylpyrene and perylenediimide chromophores at the branch point area. The spectroscopic properties of the chromophores can be modified depending on the composition of the DNA junction, ranging from monomer or excimer fluorescence to complete quenching, demonstrating the potential of such assemblies for use in diagnostic, electronic, optical and mechanical applications
The naturally occurring 3-way junction formed at the branch point of three DNA or RNA strands is a useful structure for organising functional molecules. In this hot paper Markus Probst et al. have used the three-way junction to assemble non-nucleosidic alkynylpyrene and perylenediimide chromophores at the branch point area. The spectroscopic properties of the chromophores can be modified depending on the composition of the DNA junction, ranging from monomer or excimer fluorescence to complete quenching, demonstrating the potential of such assemblies for use in diagnostic, electronic, optical and mechanical applications
![Rich_med_tcm18-153780[1]](https://blogs.rsc.org/ob/files/2012/01/Rich_med_tcm18-15378013.jpg)