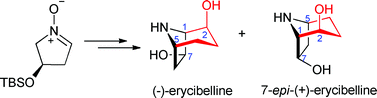
Chu-Yi Yu and colleagues present the first stereoselective synthesis of (-)-Erycibelline in this OBC hot article.
(-)-Erycibelline is a naturally occurring dihydroxynortropane alkaloid isolated from the Chinese herb medicine Erycibe elliptilimba, which is used to treat rheumatic disease and is a useful pain-killer.
Yu’s team first synthesized a cyclic nitrone with both nitrone and aldehyde functional groups as the key intermediate by organometallic addition of nitrone followed by oxidation and deprotecton. Subsequent SmI2-induced intramolecular reductive coupling and reductions produced (-)-Erycibelline with good yields.
This neat approach to the nortropane skeleton is versatile and could allow general access to hydroxylated nortropane alkaloids and their analogues, which could be beneficial for treating other diseases.
Download the article now to read more details of this elegant synthesis – it’s free to access for 4 weeks!
A concise stereoselective synthesis of (−)-erycibelline
Z.-L. Zhang, S. Nakagawa, A. Kato, Y.-M. Jia, X.-G. Hu and C.-Y. Yu
Org. Biomol. Chem., 2011, DOI: 10.1039/C1OB06244A