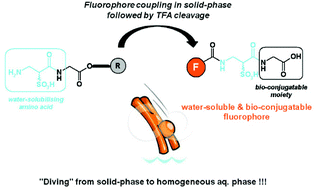
Fluorescent bio-probes, polypeptide tags and reporter proteins have become invaluable tools in life science research. So labelling of natural biological molecules with fluorescent organic dyes has become a popular area of research. But while many fluorphores perform well in organic solvents, it’s not so easy to make ones that work well the aqueous media required for biological applications.
Now, Anthony Romieu and colleagues at the University of Rouen, France, have devised an easy and efficient solid-phase synthesis to obtain rapidly water soluble choromophores and fluorophores in a highly pure form. They report the first successful use of N-Fmoc-a-sulfo-b-alanine as a solid-phase peptide synthesis building block, which could open the way to the future development of promising direct protein labelling.
To read more, download the article – it’s free to access for the next four weeks:
N-Fmoc-α-sulfo-β-alanine: a versatile building block for the water solubilisation of chromophores and fluorophores by solid-phase strategy
Anthony Romieu, Thomas Bruckdorfer, Guillaume Clavé, Virgile Grandclaude, Cédrik Massif and Pierre-Yves Renard
Org. Biomol. Chem., 2011, DOI: 10.1039/C1OB05730