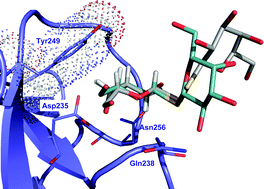
Jésus Jiménez-Barbero, CSIC, and an international team of researchers show that DTDG is capable of selecting between the mistletoe toxin and human lectins and conclude that glycosyldisulfides have potential as chemical platform for inhibitor design.
Interested? The article is free to download for the next 4 weeks:
Symmetric dithiodigalactoside: strategic combination of binding studies and detection of selectivity between a plant toxin and human lectins
Sonsoles Martín-Santamaría, Sabine André, Eliza Buzamet, Rémi Caraballo, Gloria Fernández-Cureses, Maria Morando, João P. Ribeiro, Karla Ramírez-Gualito, Beatriz de Pascual-Teresa, F. Javier Cañada, Margarita Menéndez, Olof Ramström, Jesús Jiménez-Barbero, Dolores Solís and Hans-Joachim Gabius
Org. Biomol. Chem., 2011, Advance Article
DOI: 10.1039/C0OB01235A










