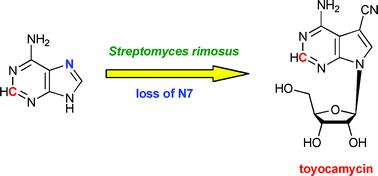
Deazapurines are bicyclic heterocycles derived from purine where one nitrogen atom is replaced by a carbon. Although they have been widely studied in recent years, the mechanistic understanding of the biosynthesis of 7-deazapurines has always been intriguing.
There is a question that inevitably arise when looking at the biosynthetic pathway of deazapurines: Where does N-7 go?
Now, Professor Moody and his group at University of Nottingham have solved this mystery by using a doubly labelled purine-adenine and following it up by NMR spectroscopy and mass spectrometry.
Their conclusions are supported and support a recent study published in Biochemistry.
If you want to find out more about the fate of this nitrogen and the mechanistic understanding of the biosynthesis of deazapurines, read this HOT paper which is free to access until 16th March.
7-Deazapurine biosynthesis: NMR study of toyocamycin biosynthesis in Streptomyces rimosus using 2-13C-7-15N-adenine
Ugo Battaglia, Jed E. Long, Mark S. Searle and Christopher J. Moody
Org. Biomol. Chem., 2011
DOI: 10.1039/C0OB01054E