Nature has been giving organic chemists inferiority complexes for many, many years, so it is always good to see a neat piece of synthesis for a tricky natural product.
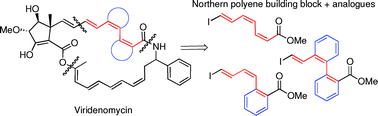
The structure of viridenomycin – an antibiotic with anti-tumor activity – has been known for 20 years, but attempts to synthesise it thus far have failed due to its complex structure and inherent instability. Now, Andy Whiting and colleagues from Durham University have synthesised fragments suitable for constructing a particularly unstable part of viridenomycin via a series of cross-coupling reactions.
They focused on the northern polyene fragment, synthesising several analogues with better isometric ratios and increased stability towards photoisomerisation than the original tetraene fragment. The authors hope that their method may help to achieve the total synthesis of this valuable complex molecule.
Read how they did it online – the article is free to access for 4 weeks!
Studies towards the synthesis of the northern polyene of viridenomycin and synthesis of Z-double bond analogues
Jonathan P. Knowles, Victoria E. O′Connor and Andrew Whiting
Org. Biomol. Chem., 2011, Advance Article
DOI: 10.1039/C0OB00977F