Ferrocene units confer a set of interesting properties to ligands for transition metal complexes, such as reversible redox- properties, planar chirality, or strong electron donating ability. While there are numerous examples of ferrocenylphosphines in which the ferrocene unit is directly bonded to the active coordination center – therefore maximizing its impact – attempts at stabilizing a carbene center using one or two ferrocenyl substituents had yet to prove successful.
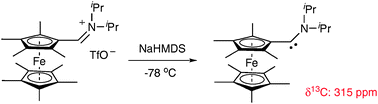
Now, Guy Bertrand and colleagues at the University of California, Riverside, have successfully prepared the first stable carbene featuring a ferrocenyl unit directly bonded to the electron-deficient center, by using a pi-electron-donating substituent in the form of an amino group. The team was able to spectroscopically characterize and chemically trap the novel (amino)(ferrocenyl)carbene compound.
Such work is envisioned to open perspectives for the synthesis of new types of carbene ligands, and will be of high relevance to researchers in fundamental chemistry, organometallics, supramolecular chemistry, catalysis and beyond. Challenges ahead now include the further stabilization of such carbenes, via the preparation of cyclic ferrocenylaldiminium systems.
A persistent (amino)(ferrocenyl)carbene
Alan DeHope, Daniel Mendoza-Espinosa, Bruno Donnadieu and Guy Bertrand
New J. Chem., 2011, Advance Article
DOI: 10.1039/C1NJ20170K, Paper
Interested? Why not read this NJC Article selected as Hot, FREE to access until 6th of June 2011.
Let us know what you think and leave us a comment below!
This article will be part of the themed issue of NJC honouring the life and work of Prof. Didier Astruc, on the occasion of his 65th birthday – Coming soon.