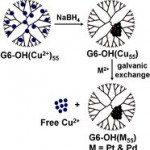
 Crooks and co-workers (University of Texas, USA) report the synthesis and characterization of Pt and Pd dendrimer-encapsulated nanoparticles (DENs) using the method of galvanic exchange. In the presence of either PtCl42− or PdCl42−, the less noble Cu DENs oxidize to Cu2+ leaving behind an equal-sized DEN of Pt or Pd, respectively. This methodology is shown to be much faster and giving higher yields than using the common synthetic route i.e. direct reduction with BH4−.Equally important, the detailed characterization presented demonstrates that the structure and composition of Pt and Pd DENs synthesized by direct BH4− reduction and galvanic exchange are indistinguishable.
Crooks and co-workers (University of Texas, USA) report the synthesis and characterization of Pt and Pd dendrimer-encapsulated nanoparticles (DENs) using the method of galvanic exchange. In the presence of either PtCl42− or PdCl42−, the less noble Cu DENs oxidize to Cu2+ leaving behind an equal-sized DEN of Pt or Pd, respectively. This methodology is shown to be much faster and giving higher yields than using the common synthetic route i.e. direct reduction with BH4−.Equally important, the detailed characterization presented demonstrates that the structure and composition of Pt and Pd DENs synthesized by direct BH4− reduction and galvanic exchange are indistinguishable.
Interested to know more? Why not download and read the article today! It’s recently been published in NJC and will be FREE to access for a period of 4 weeks.
Synthesis, characterization, and electrocatalysis using Pt and Pd dendrimer-encapsulated nanoparticles prepared by galvanic exchange
Surojit Pande, Michael G. Weir, Brian A. Zaccheo and Richard M. Crooks
New J. Chem., 2011, Advance Article DOI: 10.1039/C1NJ20083F, Paper.
Pt and Pd dendrimer-encapsulated nanoparticles prepared by galvanic exchange
13 May 2011