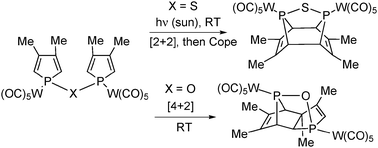
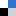A new facet of Phosphole chemistry is being uncovered it this NJC Letter by François Mathey et al. at the Nanyang Technological University, Singapore. Phosphole rings have been known for a long time to dimerize via [4+2] cycloaddition whenever the phosphorus lone pair is oxidized or complexed. The [2+2] dimerization, on the contrary, is almost unknown.
Now, the authors show that a minor change in the structure of the bisphospholes derivative – i.e. P-O-P vs. P-S-P – completely suppresses the familiar (4+2)-cycloaddition in favour of the thermally forbidden (2+2) -cycloaddition despite considerable steric constraint.
Under solar light at room temperature, the tungsten pentacarbonyl complex of the P–S–P-linked biphosphole undergoes a [2+2] intramolecular cycloaddition. Then, this [2+2] dimer gives the original [4+4] dimer via a Cope rearrangement. This sequence stands in sharp contrast with the behavior of the corresponding P–O–P-linked biphosphole which undergoes a classical [4+2] cycloaddition.
This NJC Letter has been rated as Hot, and will be FREE to access for 4 weeks. Read it now and let us know what you think!
Intramolecular [4+2] versus [2+2] cycloadditions in P–X–P-linked biphospholes (X = O, S)
Matthew P. Duffy, Yuhan Lin, Liow Yu Ting and François Mathey
New J. Chem., 2011, Advance Article
DOI: 10.1039/C1NJ20087A, Letter
This article will be part of the themed issue of NJC honouring the life and work of Prof. Didier Astruc, on the occasion of his 65th birthday – Coming soon.